An Introduction To Cell Therapy Manufacturing
Cell therapies harness the power of living cells to fight previously uncurable diseases and conditions. These groundbreaking treatments stand at the forefront of modern medicine but face unique manufacturing challenges in standardization, scalability, and regulatory compliance.
This comprehensive guide walks drug developers through the intricacies of cell therapy manufacturing, exploring its significance, best practices, regulatory concerns, and emerging technologies. This guide covers the following key topics:
- What Is Cell Therapy’s Significance In Modern Medicine?
- What Are The Differences Between Autologous And Allogeneic Cell Therapies?
- How Are Cell Therapies Manufactured?
- Why Is Cell Therapy GMP Essential?
- What Common Challenges Do Cell Therapy Manufacturers Face?
- What Innovations Are Enhancing The Cell Therapy Manufacturing Process?
- Future Directions And Trends For Cell Therapy Manufacturing
- Successful Cell Therapy Manufacturing Case Studies
- Conclusion
- Frequently Asked Questions (FAQs)
What Is Cell Therapy’s Significance In Modern Medicine?
Cell therapies treat a variety of serious conditions, including blood cancers, autoimmune disorders, and rare diseases. Manufacturers develop, produce, and deliver viable therapeutic cells through cell selection, genetic modification, expansion, and quality control. To meet regulatory approval, the final therapeutic products must be proven safe, effective, and reproducible.
Standardizing and scaling the manufacturing process is crucial for the widespread adoption of cell therapies but faces many logistical hurdles. Optimizing these processes is crucial for the industry to create accessible, affordable cell therapies and expand their use to promising new applications.
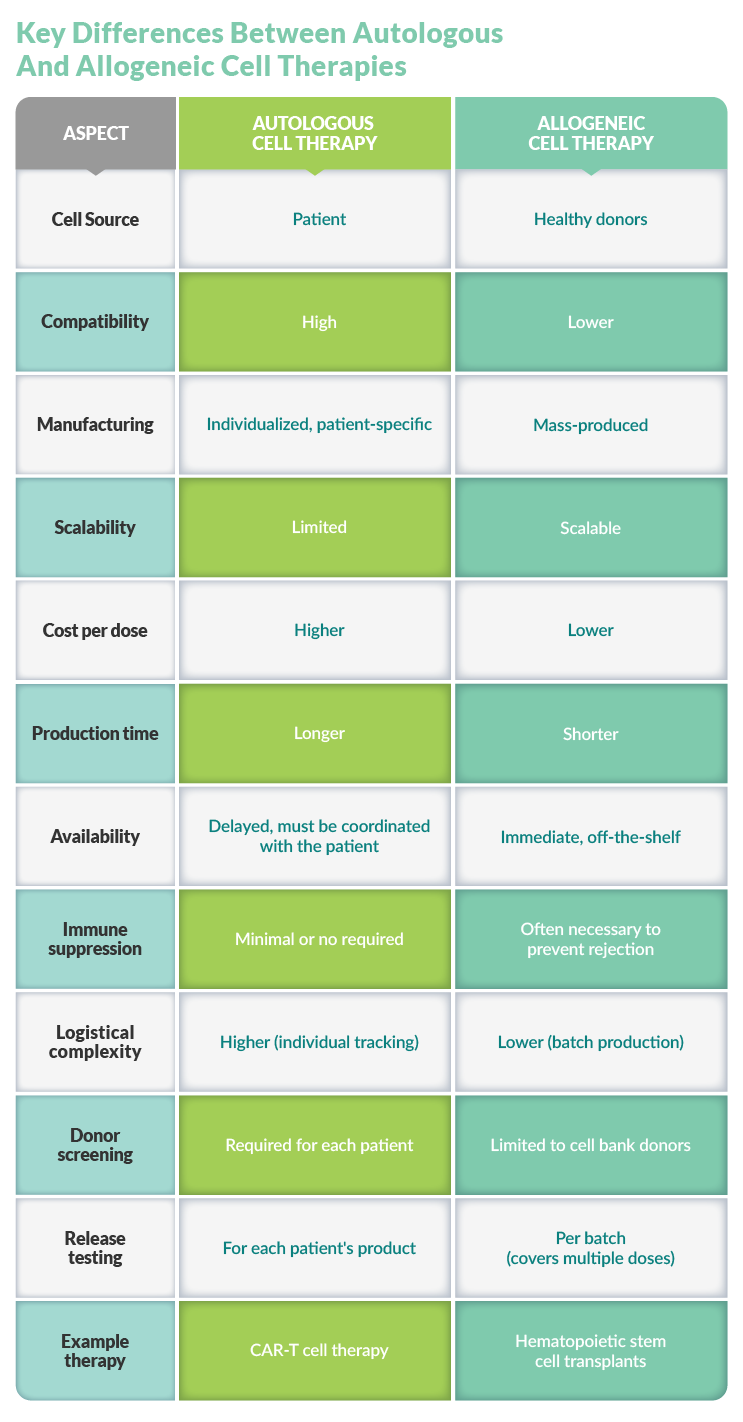
What Are The Differences Between Autologous And Allogeneic Cell Therapies?
Cell therapies are either autologous or allogeneic, depending on the target medical condition, scalability needs, and economic considerations. Both approaches have unique advantages but also carry manufacturing challenges.
Autologous Cell Therapies
Autologous cell therapies, e.g., CAR T treatments, use the patient's own cells for treatment. Process steps include cell extraction, modifying the cells in a laboratory setting, and reintroducing the modified cells to the patient.
As a personalized medicine approach, autologous cell therapy is compatible with the patient's immune system, can be tailored to the patient's particular needs, and potentially carries fewer side effects than allogeneic therapies or other types of treatments.
However, manufacturing individual treatments carries high production costs. Additionally, the process is logistically complex, as extraction, processing, and reinfusion must be carefully coordinated and fully traceable. This method is also limited by the quantity and availability of the patient’s unique cells.
Allogeneic Cell Therapies
Allogeneic cell therapies, on the other hand, use cells from healthy donors to mass-produce treatments that can reach more patients at a lower price point than autologous therapies. Additionally, sourcing cells from healthy donors can improve cell quality. However, they carry an increased risk of immune rejection, requiring careful matching plus immunosuppressive strategies.
Although allogeneic therapies are scalable, this approach also requires logistical expertise, from sourcing healthy donors to creating a final, consistent product. Robust process development and stringent quality control measures are key to meeting regulatory requirements for safety and efficacy across diverse patient populations.
How Are Cell Therapies Manufactured?
Cell therapy manufacturing is a complex, multistage process that begins with cell isolation and transitions to cell activation and expansion, sometimes using genetic engineering to modify the cells' properties to formulate the final product. Throughout the manufacturing process, cells are rigorously characterized to safeguard quality and function. Quality control testing is performed at key stages, and the final product is carefully preserved to ensure that it is still potent and viable when it reaches patients. Each step in this process is meticulously crafted to maintain cell viability, functionality, and product consistency.
Each of the cell manufacturing stages outlined below is designed to create safe, effective, and consistent cell therapy products. These processes must also be optimized and standardized to meet regulatory approval.
Cell Sourcing And Collection
First, human cells are sourced either from the patient (autologous) or a healthy donor (allogeneic). For autologous therapies, cells are typically collected via apheresis, while allogeneic therapies use available sources such as cell banks.
Cell sourcing and collection are challenging due to the following factors:
- Limited apheresis capacity: As the demand for cell therapies grows, existing apheresis centers struggle to meet cell collection quotas, creating delays.
- Standardization issues: A lack of standardized collection protocols across different sponsors and clinical trials compromises the quality of collected cells and puts pressure on apheresis units.
- Patient scheduling complexities: Coordinating patient schedules for cell collection is complex, especially in clinical trial settings. Unexpected delays due to patient health issues or travel constraints can disrupt supply chains and manufacturing processes.
- Donor matching for allogeneic therapies: Finding suitable donors for allogeneic cell therapies is complicated by immune barriers and the need for careful matching. Additionally, the lack of diverse donor pools makes it difficult for some patients to find a match.
- Quality and consistency of starting material: Ensuring the purity and consistency of collected cells, particularly for allogeneic therapies, requires specialized equipment and training. Variability in donor cells’ genetic and phenotypic characteristics affects the final product.
- Logistical constraints: Cell collection involves complex logistics, including coordinating multiple teams, e.g., apheresis staff, laboratory personnel, cryopreservation experts, and specialized couriers.
Cell Isolation
Once collected, the desired cell population (e.g., T cells) is isolated from the heterogeneous mixture to achieve maximum yield and purity. Rigorous analysis confirms the isolation procedure’s effectiveness and includes phenotype and viability assessments. Selecting the appropriate isolation technique depends on the specific cell type and downstream considerations. Standard isolation techniques include:
- Density gradient centrifugation separates based on their density.
- Magnetic-activated cell sorting (MACS) uses magnetic particles that bind to specific surface markers to isolate cells.
- Fluorescence-activated cell sorting (FACS) labels cells with fluorescent markers and sorts based on their characteristics.
Cell isolation is not a simple process, however. Low efficiency and yield limit currently available single-cell isolation techniques, leading to a shortage in starting materials for downstream processes. Furthermore, open processes and manual handling introduce potential contamination risks.
Standardization is complex because different tissues require different optimized protocols due to variations in extracellular matrix composition, cell density, and rigidity. This complexity can result in undesirable technical variations and batch effects in studies involving individual cells.
Additionally, isolation processes can induce stress on cells, potentially damaging their viability or functionality. For example, FACS can cause cell stress during sample preparation and isolation. Isolation is also costly and time-consuming, which can be a barrier to efficient manufacturing.
Cell Activation And Expansion
After isolation, cells are activated and expanded to achieve clinical or commercial relevance. Common T cell activation approaches include anti-CD3/CD28 antibodies (soluble or bead-bound), OKT3 stimulation (plate-bound or soluble), and synthetic antigen-presenting cell analogs. Other cell therapies, e.g., those involving mesenchymal stromal cells (MSCs), hematopoietic stem cells (HSCs), and other stem cell types, use a variety of methods to enhance their proliferation, differentiation, and therapeutic potential, such as:
- Growth factor supplementation: Stem cells, including MSCs, can be activated using specific growth factors. For instance, bone morphogenetic protein 3 (BMP3) enhances MSC proliferation and differentiation while transforming growth factor-beta (TGF-β) works in concert with BMP3 to activate MSCs.
- Cytokine stimulation: Granulocyte colony-stimulating factor (G-CSF) is used to mobilize and activate HSCs for transplantation.
- Chemical stimulation: Cyclophosphamide is used in combination with G-CSF to recruit and activate HSCs for transplantation.
- Metabolic regulation, such as stimulating autophagy, enhances stem cell renewal and potency. Hypoxic conditions can also activate stem cells and delay senescence.
- Signaling pathway modulation: Pharmacological regulation of specific signaling pathways can activate stem cells, including mTOR pathway modulation, PI3K/AKT pathway regulation, and Wnt/β-catenin pathway activation.
Cell culture media is another key component of cell activation and expansion. The basal media composition and serum selection are optimized for the specific cell type and target product. Additionally, supplementing exogenous cytokines promotes expansion and alters phenotype. Common examples include IL-2, IL-7, and IL-15.
Maintaining optimal density is crucial for growth and viability, and expansion timelines typically range from seven to 12 days, which affects final cell yields and phenotypes.
Metabolically, glucose and glutamine uptake patterns must be monitored, as different media lead to distinct metabolic phenotypes. For T cells, mitochondrial activity further influences their function and persistence.
Stimulation strength affects expansion and differentiation, while expansion protocols, such as rapid expansion protocols or the use of bioreactors, affect T cell expansion and phenotype.
Cell Engineering
Cell engineering enhances the therapeutic potential of many cell therapies. This process can entail genetic engineering, synthetic biology approaches, and other innovative strategies.
Several methods allow scientists to engineer cells. For instance, CRISPR/Cas9 provides precise DNA modification, introducing or deleting specific genes. Viral transduction relies on vectors to deliver genetic material into cells, while non-viral methods offer alternative genetic modification approaches.
Meanwhile, synthetic biology is revolutionizing cell therapy design. CARs direct T cells to recognize and target specific cancer antigens. T cell receptor (TCR) engineering builds on this concept, modifying receptors to recognize intracellular targets, including tumor-specific mutations. Furthermore, synthetic regulatory circuits allow engineered cells to interact with their environment and conditionally regulate their effector functions, adding a new layer of control and specificity to cell therapies.
Cell engineering extends beyond genetic modifications, however. Bioconjugation attaches molecules to cell surfaces, altering their properties or functions. Cell fusion creates hybrid cells with the desired characteristics. Emerging approaches like cell membrane wrapping and intracellular drug loading are poised to improve targeting, functionality, or therapeutic payload delivery.
Cell Characterization
Throughout the manufacturing process, cells are characterized to ensure they meet critical quality attributes (CQAs) by assessing their genomic integrity, gene and marker expression, functionality and potency, and purity and viability. Characterization takes a multifaceted approach to assess various aspects of the cells, including their identity, purity, potency, and safety, to meet regulatory standards and relies on the following techniques:
- Morphological analysis uses a microscope to examine the cell’s shape, size, and structure.
- Molecular profiling analyzes gene expression, protein production, and other molecular features.
- Functional assays assess the cells' ability to perform their intended therapeutic function.
- Phenotypic analysis via techniques like flow cytometry evaluates surface marker expression.
Cell characterization relies on advanced technologies. For example, flow cytometry provides high-throughput, multi-parameter analysis of protein expression in specific cell subpopulations. Molecular characterization techniques like DNA sequence verification identify genetic modifications and verify cell identity.
Cryopreservation During Transport And Storage
Cryopreservation maintains the final drug product’s stability and functionality until the therapy is given to the patient and entails:
- Controlled rate freezing to minimize cellular damage
- Cryoprotectants that protect cells during freezing
- Careful monitoring of temperature and storage conditions
Cells are kept at extremely low temperatures, typically below -130°C, to halt metabolic activities and preserve their integrity. They are typically in the vapor phase of liquid nitrogen, which provides prolonged storage without degradation. Solutions containing cryoprotective agents like dimethyl sulfoxide protect cells from damage during freezing and thawing. The freezing process itself is carefully controlled, usually at a rate of -1°C/minute, to minimize ice crystal formation and cellular damage.
Transporting and storying cryopreserved cell therapies necessitates specialized equipment and precise management. Cells are kept in cryogenic vials or bags designed to withstand ultra-low temperatures with protective overwraps that prevent mechanical damage. Temperature ranges must be maintained throughout shipping and storage to avoid loss.
Why Is Cell Therapy GMP Essential?
GMP systems ensure that drug products are consistently produced and controlled according to strict quality standards by scrutinizing every aspect of production, including raw materials, equipment, and staff training. GMP also requires detailed written procedures for each process that affects product quality as well as documentation that these procedures are followed to the letter. These systems prevent contamination, cross-contamination, mix-ups, and errors in manufacturing processes and are crucial to quality assurance.
Regulatory Requirements For GMP Compliance
Unlike small molecule drugs, cell therapies rely on biological materials that can vary from lot to lot, adding complications to the regulatory process. Additionally, the science behind these advanced medicines is evolving rapidly, and regulatory agencies must keep up with new manufacturing techniques. Nevertheless, regulatory bodies worldwide provide stringent GMP guidelines on cell therapy manufacturing to protect product safety, quality, and efficacy, including the FDA and EMA.
FDA Regulations
The FDA regulates cell therapy manufacturing under these guidelines:
- 21 CFR Part 1271: Covers human cells, tissues, and cellular and tissue-based products
- 21 CFR Part 600 and 610: Biologics requirements
- 21 CFR Part 211 and 212: Drug manufacturing requirements
To comply with the FDA’s requirements, manufacturers must demonstrate:
- Sterility: Manufacturers must employ aseptic processing methodologies, preferably in closed systems.
- Quality control: Clearly defined CQAs and procedures for controlling incoming materials, performing in-process testing, and conducting final product testing are required.
- Impurity control: Validated processes must be used to remove impurities like endotoxins, residual solvents, and animal products.
- Potency testing: Due to the challenges in cellular product testing, these guidelines allow some flexibility.
Recent FDA Draft Guidance
In 2023 and 2024, the FDA issued draft guidances on manufacturing cell and gene therapies testing the safety of allogeneic cells expanded for use in cell-based medical products and considerations for using human and animal-derived materials in manufacturing.
EMA Regulations
The EMA classifies cell therapies as advanced therapy medicinal products (ATMPs) and provides these guidelines:
- Directive 2001/83/EC covers medicinal products for human use.
- Regulation (EC) No 1394/2007 is specific to ATMPs.
Common Requirements Between The FDA And EMA
The FDA and EMA, as well as other global regulatory agencies, agree on the following criteria:
- Quality management systems must be in place to ensure consistent product quality.
- Risk-based approaches are critical in early-phase trials.
- Donor cells for allogeneic products must meet strict screening and testing requirements.
- Traceability via systems in place that track products from donor to recipient.
- Pharmacovigilance for ongoing safety monitoring and risk management.
Maintaining GMP Standards For Safety And Efficacy
Maintaining GMP standards necessitates a multi-pronged approach that addresses the entire manufacturing process, including validating and documenting compliance.
Documentation And Quality Control
Comprehensive documentation is a must for GMP compliance. This documentation includes detailed records of equipment specifications, construction materials, and procedures, as well as factory acceptance test reports, quality control certifications, and calibration documentation.
Risk-Based Approach
A risk-based approach to manufacturing starts with designing organizational, technical, and structural measures specific to product and process risks and implementing robust control and mitigation measures.
Long-Term Safety Monitoring
Monitoring long-term safety addresses potential concerns through prospective registry studies, long-term patient follow-ups, post-authorization safety and efficacy studies, and periodic safety update reports.
Quality Assurance
A stringent QA system covers every aspect of the manufacturing process, ensuring consistent production and routinely assessing and upgrading procedures.
Facility Requirements For Cell Therapy GMP Manufacturing
Cell therapy manufacturing facilities have specific GMP requirements that encompass many aspects of the manufacturing process and facility design.
Cleanroom Design And Classification
GMP facilities for cell therapy manufacturing must include cleanrooms, which are classified based on air purity:
- ISO classification: Cleanrooms are usually classified as ISO 7 (Class 10,000) or higher, with some areas potentially requiring ISO 6 or even ISO 5 classification.
- Air quality control: HEPA filtration systems control airborne particles and minimize contamination risks.
- Pressure differentials: Controlled air pressure is required, often with positive pressure, to prevent contamination.
Facility Layout And Flow
Appropriate layout and flow help prevent contamination. First, the unidirectional flow of materials and staff minimizes cross-contamination. Next, manufacturing, storage, and quality control testing should each be performed in distinct areas. Finally, many cell therapy facilities are designed to operate at Biosafety Level 2.
Equipment And Infrastructure
All equipment must be validated, calibrated, and maintained according to GMP standards, and specialized equipment is required, including:
- Biosafety cabinets
- CO2 incubators
- Centrifuges
- Cell culture systems (e.g., bioreactors)
- Controlled-rate freezers
- Liquid nitrogen storage systems
Environmental Monitoring And Control
Manufacturing environments must be strictly controlled, including temperature and humidity. Airborne particles in cleanrooms are also monitored, and facilities undergo regular testing for microbial contamination.
Quality Management Systems
Facilities need a comprehensive QM system for SOPs, batch records, and quality control, as well as dedicated QC labs and QA oversight. Personnel must be adequately trained in GMP procedures and cleanroom operations.
Regulatory Compliance
Facilities must be accredited by relevant regulatory bodies, such as the FDA or EMA, and undergo regular audits and inspections to ensure ongoing compliance.
Additional Considerations
Given the variability of cell-based therapeutics, facility design must be flexible and adaptable to accommodate different cell therapy processes. However, sterilization capabilities and material quarantine areas are industry standards and access to GMP areas and monitoring systems should be highly restricted.
What Common Challenges Do Cell Therapy Manufacturers Face?
Manufacturing advanced cell therapies engenders unique challenges across biological, manufacturing, and logistical domains. Cells' inherent variability presents biological complications. Manufacturing is costly, involves many manual processes that are prone to contamination, and can be difficult to scale. Logistically speaking, complicated supply chains, cold chain requirements, and, in the case of autologous therapies, patient schedules all affect production timelines.
Biological Challenges
Biological challenges are a given in cell therapy manufacturing, but addressing these challenges enables the creation of more effective and available therapeutics.
Cell Type Variability
Cells’ natural variability, particularly for autologous therapies, presents manufacturing challenges. Each patient’s cells carry unique characteristics, which unfortunately leads to inconsistencies in the final product. This variability also affects cell expansion rates, culture conditions, and overall product quality.
The diversity inherent in cell lines makes it difficult to create standardized manufacturing processes. However, manufacturers can implement mitigation strategies such as optimizing apheresis collection methods, improving target cell enrichment techniques, and developing flexible manufacturing processes that can adapt to variable starting materials.
T Cell Exhaustion
T cell exhaustion is a significant limitation in cell therapy, specifically for CAR-T cell treatments. Persistent antigen stimulation and the immunosuppressive tumor microenvironment lead to a gradual loss of T cell effector functions. The exhausted T cells are less able to proliferate and produce cytokines, limiting their anti-tumor activity. Additionally, T cell exhaustion is associated with epigenetic modifications that are not fully reversible with current therapies.
Several approaches are being explored to overcome T cell exhaustion, including modifying CAR designs to optimize signaling, targeting the transcription factors involved in exhaustion, and creating combination therapies that reinvigorate exhausted T cells.
Tumor Heterogeneity
For targeted cancer therapies, tumor heterogeneity adds a layer of complexity to treating the disease. Tumors frequently exhibit heterogeneous expression of target antigens, leading to incomplete responses. Additionally, some tumor cells lose HLA expression, evading recognition by T cells. Tumors can also evolve over time and develop resistance to single-targeted therapies.
Addressing this heterogeneity includes developing multi-targeted therapies that can target multiple antigens simultaneously, implementing clinical trial assays to assess target expression and HLA loss in patient tumors, and moving targeted therapies to earlier disease stages when tumors may be less heterogeneous.
Manufacturing Challenges
Scaling up cell therapy production while managing costs, consistency, and quality is a delicate balancing act. However, manufacturers can meet these challenges by focusing on early process development, automation, and cost-conscious strategies. As cell therapies advance, standardization and technological improvements will help make these treatments more widely accessible.
Scaling Up Production While Maintaining Consistency
Depending on the type of cell used, scaling up cell therapy production presents unique difficulties. As autologous therapies use individual patient cells, they are suited for scaling out, not scaling up. Scaling out involves processing multiple small batches, which limits economies of scale and increases production costs.
Allogeneic therapies can be created in larger batch sizes, but it’s problematic to ensure product and process consistency across different stages of development and manufacturing sites.
Manual processes and automation: Many cell therapy manufacturing processes still involve time-consuming and costly manual labor. As the field develops, automation will become the solution for handling large volumes of batches and ensuring reproducibility, especially for autologous therapies.
Quality control: As with other biopharmaceuticals, scaling up production requires maintaining strict quality control measures, which becomes more complex with increased batch sizes. Particularly for autologous therapies, each batch requires extensive documentation and testing.
Process Standardization And Cost Management
Standardizing processes and managing costs in cell therapy manufacturing is another obstacle. Cell therapy manufacturing processes are intricate and can vary significantly between different therapies. Although standardization is crucial, it must be balanced with flexibility to support unique assets and applications.
The International Organization for Standardization (ISO) and other groups are working to develop standards for cell therapy manufacturing, but a complete standardization system is still needed. Meanwhile, adhering to GMP standards and rigorously documenting procedures aids in compliance with several international regulations.
Cell therapies are expensive for patients, and major cost categories include facility operations, personnel, materials, and equipment. Manufacturers are exploring ways to optimize processes and reduce production costs.
Logistical Challenges
Due to cell therapies’ unique properties, logistics surrounding supply chain management, storage, and shipping are complex and highly regulated.
Supply Chain Complexities
Cell therapy supply chains often span multiple continents, with materials sourced from various locations worldwide. This global interdependence makes the supply chain vulnerable to disruptions from international conflicts, health crises, natural disasters, and trade disputes. In the US, the Biosecure Act is pushing pharmaceutical companies to reconfigure their supply chains.
Particularly for autologous therapies, manufacturing also demands coordination between patients, clinical sites, manufacturing facilities, and logistics providers. Delays in this intricate choreography can jeopardize the entire therapy, as there are narrow windows for collecting, modifying, and delivering cells back to the patient.
Additionally, cell therapy products are highly sensitive biological materials that require strict temperature controls throughout the entire supply chain. Cold chain logistics adds complications and costs to the transportation process. For instance, specialized packaging keeps the final product within the required temperature range during transit, while real-time temperature monitoring ensures product stability. Shipments are strictly time-bound, as delays could be catastrophic to these delicate therapeutics.
Strategies To Address Logistical Challenges
Several successful strategies mitigate these logistical difficulties, including:
- Integrated technologies like rack-and-trace systems improve supply chain coordination and visibility.
- Stakeholder collaboration between manufacturing facilities, logistics providers, and clinical sites streamlines processes and reduces delays.
- Industry-wide standardization for handling, packaging, and transporting cell therapy products improves consistency and reduces risks.
- Advanced, synchronized planning systems coordinate patient schedules, production timelines, and logistics.
What Innovations Are Enhancing The Cell Therapy Manufacturing Process?
As cell therapies expand to treat more indications, new technologies are needed to create efficient, cost-effective, and scalable manufacturing processes.
Advances In Automation And Digital Technologies
Automated and robotic systems are addressing key pain points in cell therapy manufacturing, reducing manual labor while increasing precision and reproducibility. These approaches can shorten production timelines, improve scalability, and minimize contamination risks.
Examples Of Emerging Technologies
For instance, emerging "GMP-in-a-box" systems promise to condense complex cell therapy manufacturing into compact, automated platforms by linking multiple production steps and reducing operator handling. These systems may eliminate the need for cleanrooms, reducing costs and increasing accessibility.
Data analytics and ML are increasingly used to optimize manufacturing processes, improve decision-making, and facilitate real-time monitoring of critical parameters. Additionally, second-generation automated platforms offer complete automation on a sequence of operational units, providing continuous process validation and monitoring. These platforms are fully closed, integrated, and modular for improved flexibility.
Bioreactors And Closed Systems For Expansion And Cultivation
Bioreactors are a staple in biopharmaceutical manufacturing, as they provide tightly controlled microenvironments for cell growth and development. Improvements in bioreactor technology are vital to streamlining cell therapy manufacturing, including:
- Suspension aggregate cultures: Rotating flasks, rotating wall bioreactors, stirred-tank bioreactors, and primary means for expanding embryonic stem cells or induced pluripotent stem cells.
- Closed system bioreactors: These systems provide automated media exchange and efficient waste removal, supporting the expansion of various immune cell types
The Role Of AI And ML In Process Optimization
AI and ML offer significant improvements to cell therapy manufacturing. However, many companies benefit from implementing them gradually through a series of pilot projects, building out their infrastructure and expertise over time. Once fully employed, these innovations can accelerate manufacturing in several key areas:
Process Control And Optimization
AI and ML provide real-time monitoring and control of critical process parameters, dynamic adjustment of manufacturing conditions based on live data, and prediction and optimization of complex processes like viral vector packaging.
Predictive Analytics
AI systems rapidly analyze dataset patterns to provide evidence-based insights. They can also simulate process changes without real-world risks, forecast outcomes, and facilitate proactive decision-making.
Quality Control And Consistency
AI and ML enable automated visual inspections using computer vision, detecting defects and anomalies in real-time. This reduces error rates and increases product consistency.
Codon Optimization
AI models can more precisely predict optimal codon usage for specific host organisms and expression systems than traditional optimization methods, and ML algorithms analyze vast datasets of expression data to identify patterns and correlations between codon usage and protein production levels.
Bioprocess Modeling
AI-powered digital twins and hybrid models combine mechanistic understanding with data-driven insights to predict and optimize complex processes, provide soft sensors for real-time cell concentration monitoring, and offer short-term forecasts to inform process decisions.
Automation Integration
AI and ML combined with automation create a robust system that delivers vital process control, allows for dynamic adjustments based on real-time monitoring, and helps manufacturers comply with regulatory standards.
Decentralized Manufacturing Models
Decentralizing cell therapy manufacturing helps solve some of the logistical challenges inherent in producing these products. A decentralized approach can create faster delivery times, lower transportation costs, and improve patient accessibility.
By distributing production to locations that are closer to patients and treatment centers, manufacturers lessen their reliance on costly long-distance shipping. Some decentralized models enable production at or very near the point of care, including hospitals and treatment centers. These localized approaches drastically cut down the distance between manufacturing and administration sites, which is particularly crucial for autologous therapies.
Decentralized facilities are easier to scale up or down based on local demand. Establishing manufacturing sites near patient populations also reduces the need for extensive cold chain logistics, simplifying transportation and lowering the risk of devastating temperature fluctuations. Additionally, the number of handoffs between stakeholders is slashed, decreasing delays. This method also creates redundancy in the supply chain, making it more resistant to potential disruptions.
Future Directions And Trends For Cell Therapy Manufacturing
Trends in cell therapy manufacturing aim to reduce costs while improving scalability and product quality, making these innovative treatments more accessible to patients.
Developing Accessible Allogeneic Therapies
Allogeneic therapy development is expanding because it offers greater supply chain and scheduling stability compared to autologous therapies. The industry is working to develop allogeneic therapies with durable responses and expand nonviral gene editing techniques. However, these therapies are still challenging to scale up to commercial levels.
Trends In Personalized Medicine And Gene Editing Technologies
Thanks to technologies such as CRISPR/Cas9, personalized medicine and gene editing are possible. Gene editing creates more accurate cellular and animal disease models. Additionally, induced pluripotent stem cells (iPSCs) combined with gene editing can create patient-specific disease models.
How Industry Collaboration Drives Innovation
Collaboration across the industry is vital to continuous improvement in cell therapy manufacturing. The push for standardized manufacturing processes means that companies must agree on the basic building blocks of creating cell therapies. As international regulatory frameworks become more harmonized, global collaborations may increase. Finally, partnerships between established companies and new biotechs drive investment in novel technologies.
Successful Cell Therapy Manufacturing Case Studies
The case studies described below demonstrate that cell therapy manufacturing, when combined with advanced technology and industry best practices, can successfully create lifesaving advanced therapeutics.
Accelerating Treg Therapy Manufacturing
ElevateBio partnered with Abata Therapeutics to develop a robust regulatory T cell (Treg) manufacturing process for autoimmune disease treatment when they:
- Developed a novel baseline manufacturing process in just 10 months
- Shortened the initial IND timeline for ABA-101 by approximately one year
- Successfully engineered, expanded, and manufactured thymically derived TCR-engineered Tregs as therapeutics for the first time
- Created a template process to benefit future development programs
Scaling Allogeneic CAR-T Production
Catalent implemented strategic programs to bring the first allogeneic CAR-T therapy to market, including:
- Investment in infrastructure and expertise across process development, manufacturing, and fill-and-finish
- Implementation of "Manufacturing by Design" methodologies from early development to late-stage clinical manufacturing
- Improvement of cost and labor efficiencies for partner companies
Automating Large-Scale T Cell Manufacturing
GSK and Miltenyi Biotec developed an automated process for autologous T-cell therapy products using the CliniMACS Prodigy, which:
- Achieved 1.5 × 10^10 cells after 12 days of expansion
- Created a closed, automated system compatible with cryopreserved apheresis and drug product
- Successfully transferred the robust process to three manufacturing facilities
Transitioning From Research To Clinical Manufacturing
Autolus joined CGT Catapult to scale up CAR-T cell therapy manufacturing and:
- Established GMP processes for autologous CAR-T production
- Scaled operations across three manufacturing modules to meet clinical trial demand
- Implemented higher-throughput supply chains using component-kitting systems
Creating A cGMP Biomanufacturing Center
Celularity converted a 150,000-square-foot property in Florham Park, NJ, into a purpose-built cell and biomaterial manufacturing facility. The location was ideal for access to their existing team, an available skilled workforce, and nearby hospitals for sourcing placental materials. This strategic manufacturing approach allows them to:
- Control manufacturing to protect IP and drive innovation.
- Use CRISPR/Cas9 to modify placenta-derived cells.
- Conduct clinical trials on their unmodified NK cell therapy, CYNK-001, and develop CYNK-301, an enhanced CAR-NK cell therapy.
Leveraging A Manufacturing-First Approach
Artiva Biotherapeutics met the challenges of developing a fully scalable manufacturing process for NK cell therapies with a manufacturing-first approach that encompasses:
- Building on a platform developed by their partner, GC Cell, who brought over ten years of experience in cell therapy.
- Combining preselected cord blood, engineered feeder cells, and selected media to create an efficient process.
- Establishing manufacturing capability in San Diego.
- Scaling up from 50 to 200 liters and increasing downstream processing capabilities.
Decentralizing Manufacturing With A “Beside” Approach
Elixirgen Therapeutics uses a unique "bedside" manufacturing approach that creates therapeutics for patients with dyskeratosis congenita and other telomere biology disorders. Elixirgen's process includes:
- Adopting a decentralized strategy to improve patient access and simplify logistics.
- Using Miltenyi Biotec's CliniMACS Prodigy system to manufacture autologous therapies on-site in approximately 28 hours.
Conclusion
Cell therapies promise to cure previously untreatable diseases and save lives. However, manufacturing these advanced therapeutics is a complex, delicate enterprise that can carry a high price tag. As these therapies advance, new and improved manufacturing techniques are necessary to keep up with demand. With the evolution of Pharma 4.0, cell therapies are poised to expand, becoming more affordable and accessible to patients worldwide.
For information on how gene therapies are made, visit our gene therapy manufacturing page.
Frequently Asked Questions (FAQs)
Below are FAQs regarding optimizing cell therapy manufacturing.
1. What are the key differences between autologous and allogeneic cell therapy manufacturing?
Key differences include:
- Autologous therapies require "scaling out" rather than traditional "scaling up," which involves processing multiple small batches.
- Allogeneic therapies allow for larger batch sizes and more supply chain stability.
- Autologous products are more variable due to patient-specific starting material.
- Allogeneic manufacturing can be faster, with some processes taking as little as 24 hours compared to months for autologous therapies.
2. How do regulatory challenges impact cell therapy manufacturing?
Regulatory challenges impact cell therapy manufacturing in several ways:
- Cell therapies require a separate, evolving regulatory framework as "living medicines."
- Adhering to the latest requirements is critical for swift approvals and commercialization.
- Standard methods for classical biologics are often unsuitable for cell therapies, necessitating novel approaches.
- CMC issues have led to disproportionately high clinical holds for cell therapy trials compared to other drug products.
3. What are the latest advancements in cell therapy manufacturing processes?
Recent advancements include:
- Automation and closed system manufacturing to reduce contamination risks and labor requirements.
- Integration of AI and ML for process optimization and control.
- Development of end-to-end automated platforms
- Implementation of modular and flexible manufacturing systems.
4. How is cell therapy manufacturing ensuring product purity and reproducibility?
Cell therapy manufacturers are ensuring product purity and reproducibility by:
- Implementing closed manufacturing systems to minimize contamination risks.
- Conducting in-process testing to monitor control of the manufacturing process.
- Developing adaptive manufacturing processes that can ensure final product reproducibility despite variable starting materials.
- Focusing on stringent quality control measures and defining critical quality attributes.
- Utilizing sequential enrichment steps to reduce cellular impurities and enrich target cells.
5. What role does cell engineering play in enhancing the effectiveness of cell therapies?
Cell engineering plays a crucial role in enhancing cell therapy effectiveness by:
- Enabling the creation of more accurate cellular and animal models of disease.
- Allowing for the development of patient-specific disease models using iPSCs combined with gene editing.
- Facilitating the selection of patients for gene editing therapies to correct specific mutations.
- Improving the functionality and potency of cell therapies through genetic modifications.
- Enabling the development of more targeted and efficacious cell therapies through precise genetic alterations.
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotech, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON CELL THERAPY MANUFACTURING
-
Legend's CARVYKTI Offers Lessons In Commercial-Phase Changes
The company is starting small with a pilot to do what most companies avoid altogether — innovating the manufacturing process for a product already on the market.
-
Cell & Gene Therapy Manufacturing: Considerations For Early-Stage Companies
Key considerations for early-stage cell and gene therapy companies include whether to develop and manufacture internally vs. externally, locally vs. not, and how to choose a CDMO.
-
Audit Readiness For Cell & Gene Therapy Companies
Cell & gene therapy companies are required by regulation to qualify sites performing all the steps and methods as part of their manufacturing processes.
-
CTMC's 2025 Cell Therapy Manufacturing Outlook
CEO Jason Bock looks back on milestones of 2024, namely an approved TIL for melanoma, and explains how it sets the stage for this year.
-
Hear Me Out — Cell Therapy GMP Starts With The Donor
Regulators also have indicated they expect allogeneic cell therapy donors to meet more rigid eligibility requirements and undergo screening.
EDITORIAL PERSPECTIVES ON CELL THERAPY MANUFACTURING
-
Easing Autologous Cell Collection, Automating Manufacturing
To circumvent the challenges of leukapheresis, CellProthera developed a novel method for collecting therapeutic stem cells from whole blood. CSO Ibon Garitaonandia, Ph.D., discusses the company's mission to streamline manufacturing.
-
Cell Therapy Manufacturing Trends And Advancements Continuing In 2025
In this segment of the executive roundtable discussion, our panelists reflect on recent advances in cell therapy manufacturing and discuss active areas of innovation.
-
2025 Outlook For CGT: Focus On Non-Oncology Indications, In Vivo Gene Editing, And POC
Leaders from Kiji Therapeutics, Precision Biosciences, and Orgenesis weigh in on what will likely accelerate the development of novel treatments, potentially revolutionizing patient care across a wider range of diseases and bringing the promise of personalized medicine closer to reality in 2025 and beyond.
-
Allogene's Mission To Democratize CAR-T
Allogene Therapeutics CEO and co-founder, David Chang, MD, Ph.D., talks about the exciting future of allogeneic CAR-T and shares the company's mission to bring these therapies to patients.
-
Integrating Analytics And Automation In Cell Therapy Manufacturing
Experts from last month’s Cell & Gene Live event discuss practical ways to utilize emerging technologies.
ON-DEMAND CELL THERAPY MANUFACTURING WEBINARS
-
Making MSCs GMP: A Compliant Workflow
Generate GMP-compliant mesenchymal stem cells (MSCs) at scale with a closed, automated workflow. Learn how to optimize MSC harvest yields and ensure trilineage differentiation.
-
Maximizing CAR-T Cell Yields With Perfusion And Stirred-Tank Bioreactors
Join UCL experts in exploring how advanced perfusion techniques and stirred-tank bioreactors can enhance CAR-T cell manufacturing with improved yields, scale-up, and reduced costs.
-
Revolutionizing Cell Therapy Manufacturing With AI
Discover how AI can optimize processes, improve efficiency, and enhance the understanding of cellular processes, ultimately leading to more effective and personalized patient treatments.
-
Enabling GMP Production Of sgRNA For CRISPR-Based Cell And Gene Therapies
Explore critical steps and key regulatory requirements for developing CRISPR therapeutics and gain practical advice on how to avoid common pitfalls.
-
Innovative Contamination Control: Enabling Integrity And Efficiency
An integrated approach can enhance contamination control, boost process efficiency, and ensure the production of high-quality cell therapy products.















