Cell & Gene Therapy Formulation
Cell and gene therapy (CGT) formulation designs and prepares the final therapeutic product into a stable, effective, and safe medium for patient use. Unlike traditional drugs, CGTs rely on complex, fragile biological materials like living cells, viral vectors, and nucleic acids, which require specialized strategies to preserve their potency, viability, and function.
Formulation bridges development and clinical use by protecting therapeutic components from degradation, ensuring biological function, and enabling targeted delivery. CGTs demand tailored approaches to stability, cryopreservation, excipients, and packaging that consider product and patient needs.
As CGT development advances, formulation is vital to scalability, regulatory compliance, and patient outcomes. This comprehensive guide explores CGT formulation, including:
- Core Formulation Strategies
- Quality Control And Analytical Techniques
- Automation In Formulation Processes
- Regulatory Considerations For Formulation
- Future Trends And Emerging Innovations
- Frequently Asked Questions ( FAQs)
Core Formulation Strategies
CGT formulation strategies balance product integrity with manufacturing efficiency. Given these therapeutics’ sensitive nature, CGTs require precise formulation that ensures potency and safety. When scaling from labs to commercial manufacturing, companies benefit from advanced solutions that optimize processes while meeting regulatory requirements.
Gene Therapy Formulation
A gene therapy formulation strategy considers vector selection, purification techniques, stability and shelf life, delivery efficiency, and scalability.
Selecting Vectors For Gene Delivery
Viral and non-viral vectors both transfer genes, but their transduction efficiency, immune response, and long-term expression differ by type.
Common viral vectors include adeno-associated virus (AAV), lentivirus, and adenovirus, which offer varying payload capacities, tissue targeting, and safety profiles. Overall, viral vectors provide high transduction efficiency but can trigger patient immune responses.
Non-viral vectors avoid immunogenicity and are more scalable than viral vectors but carry lower transient expression. Common non-viral vectors include lipid nanoparticles (LNPs), cationic liposomes, and polymeric nanoparticles like polyethyleneimine or poly (lactic-co-glycolic acid) (PLGA).
Gene Therapy Purification Techniques
Purification removes biological impurities like host cell proteins, DNA, and empty capsids. High purity standards are necessary for product safety, potency, and regulatory approval. Common purification techniques include:
- Ion exchange separates full/empty capsids via surface charge differences.
- Affinity chromatography provides ligand-based capture for high-purity AAV vectors.
- Ultracentrifugation uses density gradient separation for large-scale batches.
- Tangential flow filtration (TFF) provides concentration, buffer exchange, and impurity removal. Membrane optimization enhances yield and purity.
Stability And Shelf-Life Considerations
Gene therapies are highly sensitive to temperature, pH, and mechanical stress, so formulations must protect vector integrity and functionality during the entire product lifecycle, including storage and transport. Stabilizers, cryoprotectants, and optimized buffer systems extend shelf-life and minimize degradation.
For example, CAR-T drugs have 24-- to 72-hour viability windows and are often manufactured on-site. Cryoprotectants, such as dimethyl sulfoxide (DMSO) or LNPs, prevent aggregation during freezing. Unmethylated CpG dinucleotides in AAV vectors can trigger innate immune responses, reducing expression durability. Factors such as these are carefully weighed to optimize formulation strategies.
Delivery Efficiency
A drug’s efficacy is determined by how effectively the vector reaches target cells and expresses the gene of interest. Particle size, surface charge, and route of administration (e.g., IV, intramuscular) influence delivery outcomes. Optimizing vector-cell interactions ensures therapeutic payload expression and can be achieved through the following:
- Physical methods, such as electroporation parameters (pulse strength, duration), are tuned to balance transfection efficiency and cell viability.
- Vector engineering bidirectional promoters (e.g., β-actin) enable dual-gene delivery for complex disorders.
- Tissue-specific regulatory cassettes enhance on-target expression, such as liver-specific promoters.
Scalability For Commercial Production
Scaling formulations from clinical to commercial demand entails standardizing processes, selecting compatible raw materials, and ensuring robustness across large-scale manufacturing. Scalable formulations also reduce costs and streamline regulatory review.
Closed-system processing, standardized platforms like harmonized AAV production systems, and cost-effective chromatography methods suitable for high-volume batches are common methods to improve CGT scalability.
Cell Therapy Formulation
Cell therapy formulation involves optimizing media composition, washing and purifying, and cryopreservation.
Cell Culture Media Composition And Supplements
Optimized media formulations enhance cell expansion, reduce variability, and support therapeutic potency while maintaining cell viability, phenotype, and function.
Typically, the base media is serum-free to reduce variability and contamination risks. Growth factors like cytokines are added to promote cell proliferation and maintain desired phenotypes. Optimal pH is achieved via buffers, while amino acids, vitamins, and trace elements support cell metabolism. Antibiotics can be used to prevent bacterial contamination, though their use is minimized in final formulations.
Cell Therapy Washing And Purification
After expansion, cells are washed and purified to remove residual media components, cellular debris, and process-related impurities. Centrifugation, TFF, and closed-system fluid handling protect sterility and viability while meeting regulatory standards. Additionally, optimizing filter membrane selection and cell washing processes increases manufacturing efficiency.
Cryopreservation And Storage
Cell therapies require cryopreservation to withstand long-term storage and transport without losing integrity. Cryoprotectants like DMSO prevent ice crystal formation and cell damage, but their concentration and exposure time must be carefully managed to avoid cytotoxicity.
For instance, cells can be damaged if exposed to DMSO for more than 30 minutes, but pre-cooled media and precise mixing protocols mitigate this risk. Controlled-rate freezing, proper thawing protocols, and cold-chain logistics safeguard cell therapies’ post-thaw viability and function.
Quality Control And Analytical Techniques
QC tests the drug product at set points in the manufacturing process to ensure its safety and stability throughout its lifecycle.
Purity And Potency Assessment
Purity and potency are essential for CGT safety and efficacy. These assessments must be accurate, reproducible, and meet regulatory standards.
Purity tests look for contaminants like host cell proteins, nucleic acids, and endotoxins using the following methods:
- Flow cytometry identifies specific cell markers
- PCR-based assays detect genetic contaminants
- Microbiological testing checks for sterility
Potency assays evaluate the product's biological activities, such as gene therapy transduction efficiency or cell therapy functionality, through techniques such as:
- Functional assays measure cell activity or gene expression
- Co-culture methods assess cell-cell interactions
- Quantitative PCR calculates transgene expression levels
Stability Testing And Batch Release Criteria
Stability testing verifies that CGT products maintain their quality and efficacy over time under defined storage conditions. Physical, chemical, and functional stability are assessed at multiple points in the product’s lifecycle, including at the master cell bank and the end of production. These tests may use surrogate materials from healthy donors for initial studies.
Batch release criteria combine stability data, sterility, identity, potency, and safety testing to ensure each lot meets strict quality standards before patient administration. These quantify cell viability, count, purity, potency, sterility, and endotoxin levels.
Automation In Formulation Processes
Automation improves formulation processes by reducing contamination risks and providing real-time tracking and data capture.
Closed-System Technologies
Closed-system manufacturing enables aseptic processing and is ideal for CGT workflows that require sterility and are often created in small batches. Automated closed systems eliminate manual handling errors and reduce lot-to-lot variability, ensuring consistent quality across batches.
Separating processes like cell isolation, washing, and formulation significantly reduce contamination risks. Additionally, closed systems lower manufacturing costs by reducing extensive cleanroom infrastructure needs.
Single-Use Disposables
Single-use technologies (SUS) reduce cleaning validation burdens, lower cross-contamination risks, and improve turnaround time between batches. Disposable bags, connectors, and tubing support modular, flexible production environments suitable for personalized therapies.
Electronic Data Capture And Traceability
Digital tools streamline documentation, ensure regulatory compliance, and improve traceability across the CGT lifecycle. Automated electronic data capture (EDC) supports real-time monitoring, accurately matching personalized therapies to their intended patient by documenting every step in the drug’s journey, from material collection to final administration. EDCs also seamlessly connect interrelated systems, reducing human error and improving process reproducibility.
Electronic batch records and audit trails enhance transparency and enable faster root-cause analysis when deviations occur. Robust traceability supports good manufacturing practices (GMP) standards by providing evidence of end-to-end control over manufacturing processes.
Regulatory Considerations For Formulation
Regulatory bodies like the FDA and EMA have strict criteria for CGTs. Although they differ in the details, regulations commonly include current GMP (cGMP) compliance, QC measures, and thorough documentation requirements.
cGMP Compliance
Formulation processes must meet cGMP standards for product quality, safety, and consistency. cGMP compliance includes validated procedures, controlled environments, trained personnel, and rigorous documentation. The FDA, EMA, and other regulatory agencies enforce cGMP compliance.
Quality Control Measures
Comprehensive QC systems monitor and verify formulation processes and final product characteristics. Regulatory bodies require QC systems, including in-process testing, to track critical parameters during manufacturing, environmental monitoring, and validated analytical methods for potency, purity, sterility, and identity. Key tests measure:
- Cell viability
- Cell number/dose
- Identity
- Purity
- Potency
- Sterility and endotoxins
Novel rapid testing methods, like PCR-based sterility assays, are being developed to address time constraints.
Documentation Requirements
Thorough documentation, including standard operating procedures, batch records, quality control data, deviation reports, and traceability logs, is essential for regulatory submission and audit readiness. Accurate and complete records support transparency, reproducibility, and product approval.
Future Trends And Emerging Innovations
Delivery methods are a significant factor in CGT formulation, as they directly impact the final product’s safety and efficacy. Innovations in delivery methods are crucial to expanding CGTs to new treatment areas.
Innovations In Delivery Systems
Targeted Delivery Methods
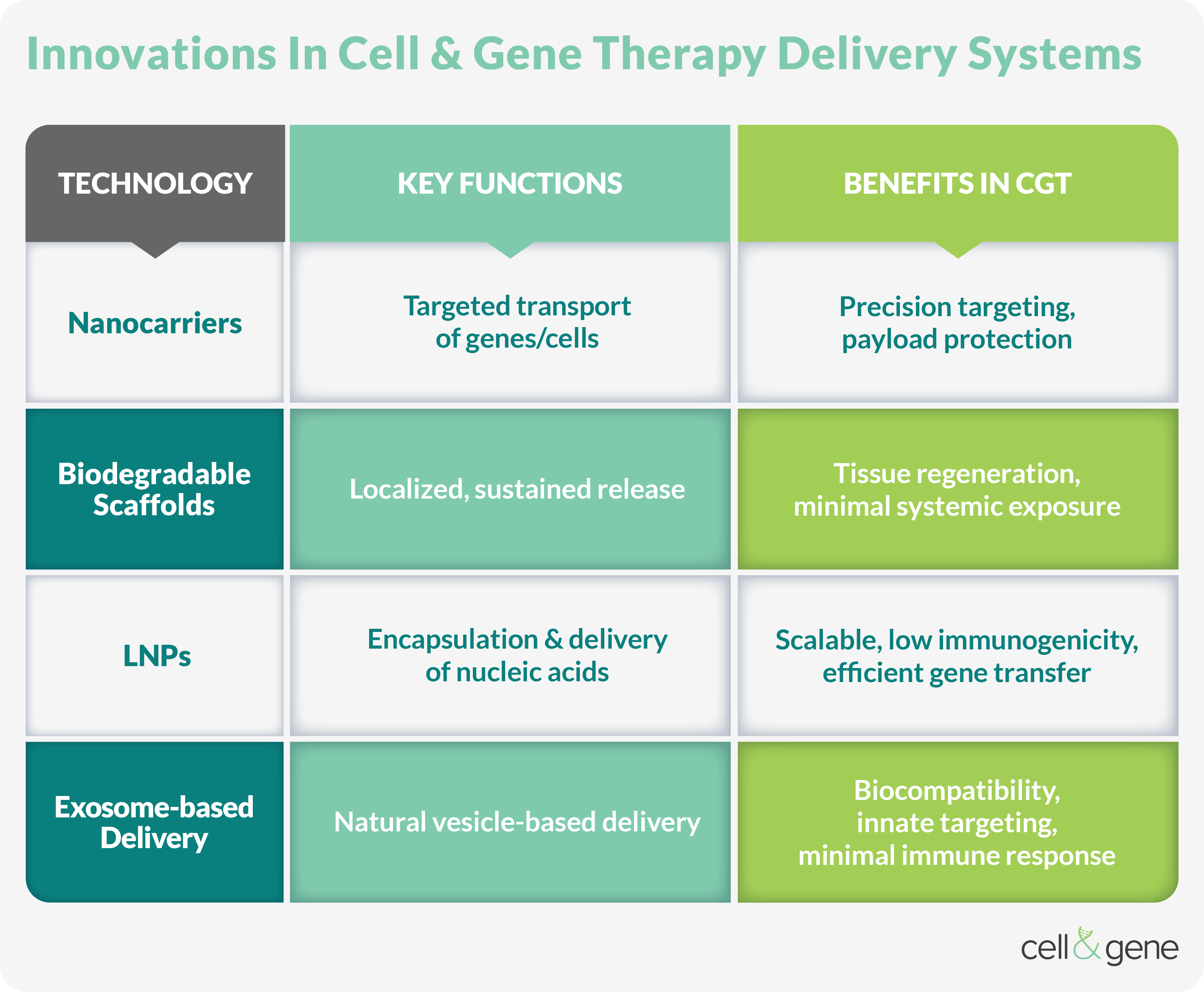
Targeted delivery methods enhance GCTs' precision, efficacy, and safety by directly delivering therapeutic agents to specific tissues or cells. Nanocarriers and biodegradable scaffolds represent significant advancements in drug delivery systems.
Nanocarriers include liposomes, dendrimers, and polymeric nanoparticles that encapsulate nucleic acids or proteins and typically employ surface modifications like ligands or antibodies for specific targeting. Nanocarriers protect payloads from enzymatic degradation and can be engineered for controlled release. Standard nanocarrier methods include:
- Folate receptor targeting: Folic acid-modified carriers (liposomes, gold nanoparticles) exploit folate receptor overexpression in cancers, enhancing tumor-specific uptake.
- Liver-targeted systems: Galactose- or lactose-modified carriers bind to asialoglycoprotein receptors in hepatocellular carcinoma.
- Sigma receptor targeting: Anisamide-conjugated systems target brain, colon, and breast cancers overexpressing sigma receptors.
Biodegradable scaffolds are three-dimensional structures composed of biocompatible materials, like PLGA or collagen, implanted at the disease site for local, sustained therapeutic delivery. They support tissue regeneration and gradually degrade after use. Additionally, tunable degradation rates and MRI-monitored drug release enable therapeutic delivery to be spatially controlled.
The Role Of LNPs In Non-viral Gene Delivery
LNPs are crucial to non-viral gene delivery, particularly for nucleic acids such as mRNA, siRNA, and CRISPR components. LNPs consist of ionizable lipids, cholesterol, phospholipids, and PEG-lipids, forming a stable structure capable of encapsulating genetic material. They facilitate cellular uptake and enable endosomal escape, delivering the genetic payload into the cytoplasm.
LNPs offer advantages over viral vectors, including lower immunogenicity, scalable manufacturing, and no risk of insertional mutagenesis. Clinically, they have been successfully used in mRNA vaccines and are being developed for broader CGT applications.
Advances In Exosome-based Therapies
Exosomes are naturally secreted extracellular vesicles and promising delivery vehicles for CGTs. As they are derived from cells, they are inherently biocompatible and unlikely to provoke immune responses. Their origin cell determines their tissue tropism and engenders targeted delivery to specific sites.
Exosomes are highly versatile and can transport many biomolecules, including mRNA, miRNA, proteins, and gene-editing tools. Various engineering techniques, such as passive loading or electroporation, can be customized to enhance cargo loading, targeting, and circulation time. Exosomes are a promising platform for regenerative medicine, cancer therapy, and precision gene delivery with potentially fewer safety concerns compared to synthetic or viral vectors.
Combination Delivery Systems
Integrating multiple technologies can enhance CGT formulations' therapeutic potential. Hybrid platforms include pairing LNPs with biodegradable scaffolds, encapsulating gene-editing tools within exosomes, or co-delivering different vector types to achieve complementary effects. For example, LNPs embedded in scaffolds provide systemic stability and localized release, while engineered exosomes coated with targeting ligands improve tissue specificity and intracellular delivery.
Combining systems leverage each delivery modality's advantages, improving the therapeutic index, and expanding treatment targets. Such strategies are especially valuable for complex or heterogeneous tissues where single-method delivery may be insufficient.
Conclusion
CGT formulation bridges the gap between scientific advancement and patient care to construct innovative treatments for an ever-expanding roster of serious diseases and conditions. However, manufacturers must balance safety and efficacy with scalability and regulatory compliance. Emerging technologies are working to make these therapeutics safer, more effective, and more accessible.
For information on other aspects of the CGT production process, visit our pages on cell therapy manufacturing, gene therapy manufacturing, and fill-finish.
Frequently Asked Questions ( FAQs)
Below are frequently asked questions on cell and gene therapy formulation.
1. What are the primary considerations when choosing between viral and non-viral delivery systems?
Key factors include immunogenicity, payload capacity, targeting efficiency, scalability, and genomic integration risk. Viral vectors often offer higher transduction efficiency but carry safety concerns, while non-viral methods like LNPs and exosomes are more versatile and safer for repeated administration.
2. What strategies ensure viral vector stability during scaling?
Optimized buffer formulations with cryoprotectants and stringent temperature control mitigate degradation. Ultracentrifugation and chromatography refine vector purity, reducing immune-triggering contaminants.
3. How do FDA guidelines address CMC challenges for viral vector-based therapies?
The FDA mandates rigorous vector identity/potency characterization, including genome integrity assays and transduction efficiency metrics. The guidance emphasizes process validation for closed-system manufacturing to limit adventitious agents.
4. What are the advantages of closed system technologies in CGT manufacturing?
Closed systems reduce contamination risk, improve product consistency, and support aseptic processing — critical factors for CGT manufacturing. They also facilitate regulatory compliance and enable safer handling of patient-specific therapies.
5. How do single-use disposables impact CGT production workflows?
SUS streamline cleaning validation, reduce cross-contamination, and increase flexibility in multiproduct facilities. They enable rapid scale-out for personalized therapies and lower overall operational costs.
6. What is the role of EDC in CGT development and manufacturing?
EDC systems enhance CGT workflows' data integrity, traceability, and compliance. They enable real-time monitoring, efficient documentation of critical parameters, and better integration across clinical and manufacturing processes.
7. How do LNPs enhance mRNA delivery in CGT?
LNPs protect mRNA from enzymatic degradation, facilitate uptake into target cells, and enable endosomal escape for cytoplasmic release. Their tunable lipid composition allows for the optimization of delivery efficiency and biodistribution.
8. What is the role of biodegradable scaffolds in local CGT delivery?
Biodegradable scaffolds enable localized, sustained release of therapeutic agents, supporting tissue regeneration while minimizing systemic exposure. Their composition and degradation rates can be tailored to specific clinical applications.
9. How are exosomes engineered to improve therapeutic delivery?
Exosomes can be loaded with therapeutic cargo through passive incubation or active methods like electroporation. Surface modifications enable targeted delivery, while manipulation of parent cells can influence exosome content and tropism.
10. What challenges remain in developing targeted delivery methods for CGT?
Major challenges include achieving high-efficiency tissue-specific targeting, avoiding off-target effects, overcoming biological barriers like immune clearance, and scaling up production under GMP conditions.
11. Can combination delivery systems improve CGT outcomes?
Yes, integrating multiple delivery strategies — such as using LNPs within scaffolds or combining exosomes with targeting ligands — can enhance delivery precision, durability, and therapeutic response while reducing side effects.
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotech, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON CGT FORMULATION
-
The Great Cell Therapy Reset: Solving The Industrial Math Of Living Drugs
The cell therapy sector is experiencing a paradigm shift in manufacturing: from bioreactor (ex vivo) to body (in vivo).
-
In Vivo LNP-Engineered Cytokine-Armored CAR Cells For Solid Tumors
By injecting lipid-based nanoparticles encapsulating mRNA and encoding the CAR directly into the bloodstream, developers can effectively reprogram the patient's own immune cells in situ.
-
Powering Cell Therapies With RNA: A New Code For Engineered Immunity
RNA is redefining cell therapy engineering—enabling transient, programmable control of immune and stem cells while simplifying manufacturing, improving safety, and accelerating scalable, virus-free workflows.
-
Strained Manufacturing, Complexity Stymie In Vivo Progress
Explicitly mapping delivery challenges to the associated GMP process steps converts biological risk into operational risk.
-
To Fight Cancer, Gene Editing Needs To Solve Its Delivery Problem
The choice of delivery dictates the complexity of manufacturing, which in turn determines the eventual cost and accessibility of the therapy.
ON-DEMAND CGT FORMULATION WEBINARS
-
Five Practical Considerations To Move From Concept To Clinic
Learn more about five key factors for advancing targeted in vivo LNP programs, from formulation and targeting strategies to bioanalytical readiness and scalability.
-
Defining Specificity Within The ELISpot Assay
Achieving high-quality T cell ELISpot results requires robust controls to verify antigen-specific activation. Learn the experiments and best practices for establishing and confirming assay specificity.
-
Hear From The Experts: LNPs Driving The Future Of Cell Therapy
Explore how lipid nanoparticles are driving breakthroughs in cell therapy, from mRNA-based immune cell engineering to genome editing for CAR T cells and HSPCs from leading experts.
-
Addressing The Challenges Of LNP Upscaling
Overcome LNP upscaling challenges by leveraging fluid mechanical principles. Learn how Impingement Jets Mixing achieves reproducible turbulence and consistent LNP quality across all production scales.
-
Accelerate Your pDNA And mRNA Process Development
Explore a structured approach to optimizing and scaling plasmid DNA production using E. coli, with insights into key growth parameters, benchtop feasibility, and pilot-scale performance.