Vectors In Cell And Gene Therapy
Without vectors, cell and gene therapies (CGTs) would be impossible. Vectors deliver genetic material to cells, modifying or correcting defective genes and targeting genetic disorders, cancers, and other diseases. Scientists first theorized that viruses could be used as gene therapy vectors as early as the 1960s, but the first successful vectors were developed in the 1980s. In the 21st century, advances in viral vector manufacturing and evolution have greatly improved these therapies’ safety and efficiency. Today, both viral and non-viral vectors drive gene therapy innovations and expand their therapeutic scope. This comprehensive guide to CGT vectors includes:
- Fundamentals Of Vectors In CGTs
- Types Of Vectors Used In CGTs
- Comparison Of Viral And Non-viral Vectors In CGTs
- Vector Engineering And Optimization
- Regulatory Considerations
- Challenges And Limitations In Viral Vector Therapy
- Emerging Technologies In Vector Development
- Frequently Asked Questions (FAQs)
Fundamentals Of Vectors In CGTs
Vectors introduce, modify, or replace genetic information in a patient's cells to correct genetic mutations, enhance immune responses, or produce missing or deficient proteins, treating genetic disorders, cancers, and degenerative diseases. A vector’s efficiency, safety, and specificity determine the therapy’s success.
Vectors are broadly categorized into viral and non-viral, each with advantages and limitations. Viral vectors include lentiviruses, adeno-associated viruses (AAV), and adenoviruses. These tools offer high transduction efficiency but pose risks, including immune responses or insertional mutagenesis.
In contrast, non-viral vectors, including plasmid DNA, lipid nanoparticles (LNPs), and polymer-based systems, offer safer alternatives without the risk of viral pathogenicity but often have lower delivery efficiency. The choice of vector depends on the specific therapeutic needs as well as cell type, expression duration, and safety profile.
Types Of Vectors Used In CGTs
Both viral and non-viral vectors are essential to CGT development. While viral vectors are highly efficient, non-viral alternatives are often safer and more scalable.
Viral Vectors
Viral vectors are powerful tools for CGTs but pose significant risks, such as immune responses and insertional mutagenesis. Various viral vectors are used, and selection depends on the therapeutic application.
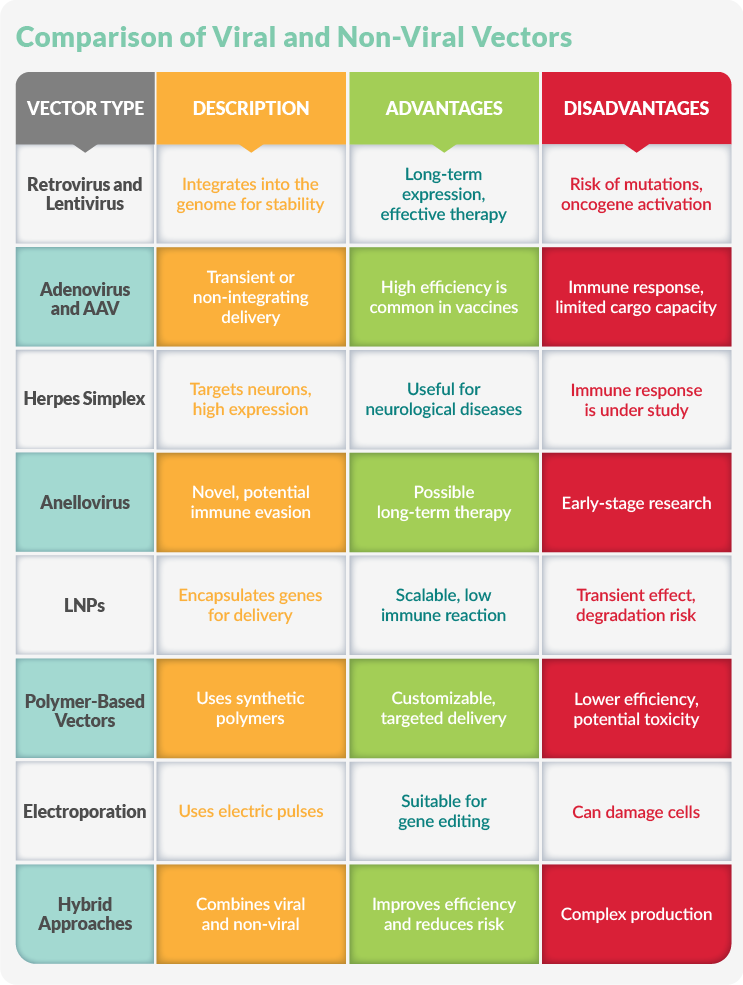
Retrovirus And Lentivirus Vectors
Retroviral vectors integrate their genetic material into the host genome for long-term gene expression. Lentiviral vectors, a subclass of retroviruses, transduce both dividing and non-dividing cells, expanding their applicability. These vectors are commonly used in hematopoietic stem cell gene therapy and CAR T cell engineering.
Adenovirus And AAV Vectors
Adenoviral vectors deliver large genetic payloads via transient gene expression, commonly for vaccine development or cancer therapy. However, AAV vectors do not integrate into the host genome, reducing the risk of insertional mutagenesis. AAV is widely used in gene therapy for inherited disorders such as spinal muscular atrophy and retinal diseases.
Herpes Simplex Virus (HSV) And Cytomegalovirus (CMV) Vectors
HSV vectors have a natural affinity for the nervous system and can target neurons. They can also carry large genetic cargo and may be used to treat neurological disorders like Parkinson's disease. CMV vectors have strong promoter activity, ensuring high levels of gene expression and making them promising candidates for vaccine and gene therapies.
Anellovirus
Anellovirus vectors are a novel category with potential advantages in immune evasion and stable gene delivery. Although still in the early research stages, they may provide an alternative for long-term gene therapy with reduced immunogenicity.
Non-Viral Vectors
Non-viral vectors exhibit lower transfection efficiency than viral vectors but avoid viral pathogenicity and immunogenicity risks.
Lipid Nanoparticles
LNPs encapsulate genetic material and deliver it to cells, providing a scalable and effective gene delivery platform. They are also widely used for mRNA delivery.
Polymer-Based Vectors
Synthetic polymers like polyethyleneimine (PEI) and poly(lactic-co-glycolic acid) form nanoparticles for gene delivery. These vectors are being refined for improved efficiency and targeted delivery.
Electroporation
Electroporation uses short electrical pulses to create temporary pores in the cell membrane, allowing genetic material to enter. This method is particularly useful for ex vivo gene editing applications, such as engineering immune cells for cancer therapy.
Hybrid Approaches
Hybrid vector systems combine viral and non-viral strategies to optimize gene delivery. For instance, synthetic nanoparticles can be engineered to carry viral components, improving transfection efficiency while minimizing immunogenicity.
Comparison Of Viral And Non-viral Vectors In CGTs
While viral vectors are highly efficient, non-viral vectors are safer, more flexible alternatives.
Strengths And Limitations Of Viral Vectors
Viral vectors offer high transduction efficiency, effectively delivering genetic material into target cells. They work for long-term or transient gene expression and treat genetic disorders and cancer. Additionally, viral vectors can be engineered to target specific cell types, enhancing their therapeutic potential.
However, viral vectors come with limitations and risks. First, they can trigger immune responses, damaging their effectiveness and safety profile. Some viral vectors also carry the risk of insertional mutagenesis, which disrupts normal gene function, creating unintended consequences. Viral vectors also are limited in the amount of genetic material they can carry. Finally, manufacturing these vectors is costly and complex.
Strengths And Limitations Of Non-Viral Vectors
Non-viral vectors are safer, more cost-effective alternatives to viral systems. First, they elicit only minimal immune responses and are easier to produce than their viral counterparts. Additionally, non-viral vectors can accommodate larger genetic payloads and deliver complex or multiple genes. This flexibility makes them suitable for personalized medicine and gene editing technologies.
Despite these benefits, non-viral vectors have lower transfection efficiency than viral vectors. Gene expression duration is also usually shorter, requiring repeated administrations for sustained therapeutic effects. Additionally, non-viral vectors are less able to precisely target specific cells. Supplementary delivery enhancements, such as chemical modifications or hybrid systems, are often necessary to improve their efficacy.
Vector Engineering And Optimization
Optimizing viral and non-viral vectors is crucial to meeting each type’s challenges expanding CGT development.
Viral Vector Capsid Engineering And Vector Modification
Viral vector capsid engineering improves gene therapy delivery, increases transduction efficiency, and ensures precise tissue targeting. Modifying viral capsid proteins enhances cellular entry while minimizing off-target effects. Directed evolution of capsids subjects viral libraries to iterative selection in specific tissues, identifying variants with superior tropism and transduction efficiency.
Specific modifications, such as peptide insertions or capsid region swaps among serotypes, alter receptor binding affinities, ensuring selective infection of target cell types. Engineered AAVs target the central nervous system, liver, or muscle for diseases affecting these organs. Tailored capsids enhance gene delivery efficiency, reduce vector dose requirements, and increase the overall safety of CGT applications.
Immune responses against viral capsid proteins limit viral vector-based gene therapies’ efficacy and longevity. Capsid engineering strategies work to reduce immunogenicity and evade pre-existing immunity. Deimmunization alters immunodominant epitopes within the capsid, preventing recognition by neutralizing antibodies or cytotoxic T cells.
Using novel or less prevalent serotypes also lowers the likelihood of pre-existing immunity in previously exposed patients. Modifications such as glycosylation, PEGylation, or stealth variant designs minimize antigenic region exposure, shielding the capsid from immune detection. Reduced immune activation extends the therapeutic window, enables repeat dosing, and improves patient tolerance.
Non-Viral Vector Optimization
Non-viral vector optimization improves their stability and efficiency. Unlike viral vectors, which naturally possess cell entry and genome integration mechanisms, non-viral vectors require structural modifications to protect genetic material and ensure effective cellular uptake. Stability improvements to nanoparticles, lipid-based carriers, or polymeric systems prevent enzymatic or immune response. For example, cationic lipids and PEI condense nucleic acids into stable, degradation-resistant complexes.
Additionally, stimuli-responsive materials, like pH-sensitive or biodegradable polymers, control the release of genetic cargo within the target cells. Optimizing particle size, charge, and surface modifications facilitates cellular uptake and endosomal escape, preventing premature lysosome degradation. These advancements make non-viral vectors reliable, viable alternatives to viral-based gene therapies.
Effective gene delivery precisely targets specific tissues while minimizing off-target effects. Non-viral vectors receive surface modifications that enable receptor-mediated uptake by desired cell types. Conjugating ligands such as peptides, antibodies, or aptamers to nanoparticles or lipid-based carriers ensures selective binding to receptors overexpressed in target tissues. For instance, folate-conjugated liposomes target cancer cells with high folate receptor expression, while transferrin-modified nanoparticles improve brain-targeted delivery through receptor-mediated transcytosis.
Another strategy uses external stimuli, such as ultrasound, magnetic fields, or light-sensitive molecules, to guide non-viral vectors to precise locations within the body. By refining targeting mechanisms, non-viral vectors enhance therapeutic outcomes while reducing systemic toxicity and immune activation.
Hybrid And Chimeric Vector Systems
Hybrid and chimeric vector systems combine the strengths of viral and non-viral vectors to create efficient and versatile gene delivery platforms. Viral vectors offer high transduction efficiency and stable gene expression, while non-viral vectors provide enhanced safety, reduced immunogenicity, and greater cargo capacity. By integrating these advantages, hybrid systems improve therapeutic potential while overcoming each vector type's limitations.
One approach encapsulates viral particles within synthetic lipid or polymeric carriers, shielding them from immune detection and enabling controlled release. Another strategy utilizes viral components, such as capsid proteins or envelope glycoproteins, to enhance non-viral carriers' cellular uptake and endosomal escape. For example, combining AAV capsids with lipid nanoparticles improves stability and targeted delivery while minimizing immune responses. Similarly, chimeric vectors engineered by swapping structural elements from different viral serotypes enhance tissue specificity and transduction efficiency.
Hybrid systems also provide tunable gene expression by incorporating regulatory elements from both viral and non-viral platforms. For instance, integrating viral promoter sequences into non-viral plasmids enhances transcriptional activity, while incorporating episomal replication elements from viruses improves transgene expression persistence.
Regulatory Considerations
Regulatory bodies like the FDA have strict requirements for vector production to protect patient safety and ensure therapeutic potency and efficacy.
FDA Guidance For Vectors
The FDA provides guidelines to ensure vectors' safety, efficacy, and quality. Vector characterization, manufacturing standards, preclinical evaluations, and clinical trial oversight are subject to stringent regulations. Developers must demonstrate vector stability, purity, and functional integrity while ensuring compliance with good manufacturing practice (GMP) regulations.
Manufacturing And Quality Control Considerations
Manufacturing viral and non-viral vectors requires strict QC measures to ensure product consistency, purity, and potency. Viral vector production uses cell culture-based systems, which must be carefully monitored for batch-to-batch variability, contamination risks, and vector yield.
Non-viral vector manufacturing focuses on nanoparticle formulation reproducibility, plasmid purity, and delivery system stability. QC tests, such as sterility assays, endotoxin testing, and potency evaluations, are necessary to meet regulatory requirements and maintain therapeutic effectiveness.
Safety Assessments For Viral Vs. Non-viral Vectors
Viral vector safety assessments evaluate immunogenicity, insertional mutagenesis risks, and potential replication-competent virus formation. Extensive in vitro and in vivo studies test host immune responses, vector biodistribution, and long-term transgene expression. While generally safe, non-viral vectors must still be tested for cytotoxicity, off-target effects, and degradation products.
Challenges And Limitations In Viral Vector Therapy
Viral vectors are the backbone of many gene therapies. However, they carry significant limitations and challenges.
Immunogenicity And Pre-Existing Immunity
The human immune system can recognize viral capsid proteins and eliminate the vector before effective gene delivery occurs. Using less prevalent serotypes, capsid engineering, and immunosuppressive treatments can mitigate these immune responses.
Vector Genome Capacity And Off-Target Effects
Viral vectors' limited genome capacity restricts their usefulness. For example, AAVs have a cargo limit of approximately 4.7 kb, which means they cannot carry large genes. Additionally, off-target effects can occur when the vector integrates into unintended genomic locations, leading to mutagenesis or unwanted gene expression. Advances in vector engineering aim to expand genome capacity and improve targeting specificity.
Long-Term Gene Expression And Stability Concerns
Maintaining stable and long-term gene expression remains a challenge, particularly for non-integrating viral vectors like AAVs, which exist episomally and may be diluted during cell division. Integrating vectors like lentiviruses provide longer-lasting gene expression but risk insertional mutagenesis. Researchers are developing self-regulating and tissue-specific promoter systems to enhance transgene stability while minimizing risks.
Vector Dose Optimization And Repeated Administration
Determining the optimal vector dose is critical to achieving therapeutic efficacy while avoiding toxicity. High doses may lead to excessive immune activation and adverse effects, while low doses can result in insufficient gene expression. Additionally, neutralizing antibodies limit repeated administration. Transient immunosuppression, vector re-administration with different serotypes, and non-viral hybrid approaches address these challenges and improve treatment outcomes.
Emerging Technologies In Vector Development
Extracellular vesicles (EVs) offer unique advantages over viral and non-viral vectors. These naturally occurring membrane-bound vesicles, including exosomes and microvesicles, facilitate intercellular communication by transporting proteins, lipids, and nucleic acids. They efficiently transfer genetic material while avoiding significant immune responses, making them an attractive alternative.
EVs also provide a biocompatible and non-immunogenic platform for gene delivery. Unlike viral vectors, they do not provoke strong immune reactions, reducing the risk of neutralization or toxicity. Additionally, EVs can be engineered to enhance targeting specificity by modifying their surface proteins to recognize and bind to specific cell types. This targeted approach minimizes off-target effects and improves therapeutic efficacy.
Another significant advantage of EVs is their ability to cross biological barriers, such as the blood-brain barrier, which poses a considerable challenge for many therapies. Researchers are exploring ways to load EVs with therapeutic RNA, DNA, or CRISPR components to facilitate precision gene editing with minimal side effects.
By combining the benefits of natural biocompatibility, immune evasion, and efficient gene delivery, EV-based vectors offer a promising third vector option. Ongoing research and optimization efforts seek to enhance their cargo-loading capacity, precision, and scalability.
Conclusion
Vectors brought CGTs from an idea to reality and introduced a new class of groundbreaking drugs to patients. Viral and non-viral vectors have limitations, but hybrid solutions and EVs may usher in the next generation of CGTs. As scientists discover more genetic solutions to serious diseases and conditions, vector development will continue to drive CGT research.
For more information on how vectors fit into the CGT manufacturing process, visit our pages on cell therapy manufacturing, gene therapy manufacturing, formulation, fill-finish, and cold chain management.
Frequently Asked Questions (FAQs)
Below are frequently asked questions about CGT vectors.
1. What is a gene therapy vector?
A gene therapy vector is a delivery system that introduces genetic material into cells to correct genetic disorders or treat diseases. Vectors can be viral (such as AAVs and lentiviruses) or non-viral (such as lipid nanoparticles and extracellular vesicles).
2. What are the main differences between viral and non-viral vectors?
Viral vectors provide high transduction efficiency and stable gene expression but may trigger immune responses. Non-viral vectors, while safer and less immunogenic, often face challenges with cellular uptake and transgene stability.
3. How do hybrid vectors improve gene therapy?
Hybrid vectors combine viral and non-viral vector elements to enhance safety, targeting specificity, and transduction efficiency while reducing immunogenicity.
4. What are the main differences between retrovirus and lentivirus vectors?
Retrovirus and lentivirus vectors are both used for gene delivery, but lentiviruses can transduce non-dividing cells, making them more versatile for gene therapy applications, whereas retroviruses require actively dividing cells.
5. How do adenovirus vectors compare to AAV vectors in gene therapy?
Adenovirus vectors provide high transduction efficiency and strong transient gene expression but often trigger significant immune responses. With a smaller genome capacity, AAV vectors offer longer-lasting gene expression with lower immunogenicity, making them more suitable for specific therapeutic applications.
6. What are the latest advancements in vector engineering for gene therapy?
Recent advancements include capsid engineering to improve tissue specificity, developing hybrid and chimeric vectors, improvements in non-viral vector formulations, and genome-editing technologies like CRISPR delivered through optimized vector platforms.
7. How do non-viral vectors differ from viral vectors regarding efficiency and safety?
Non-viral vectors are generally safer and less immunogenic but often have lower transduction efficiency than viral vectors. However, ongoing research in lipid nanoparticles, polymeric carriers, and extracellular vesicles aims to enhance their delivery capabilities.
8. What are the most common applications of gene therapy using viral vectors?
Viral vectors are commonly used in the treatment of genetic disorders (such as hemophilia and spinal muscular atrophy), cancer immunotherapy, and regenerative medicine. They enable targeted gene correction, immune modulation, and long-term therapeutic effects.
9. What challenges exist in viral vector gene therapy?
Challenges include immune responses, limited genome capacity, off-target effects, and issues with long-term gene expression and repeated administration.
10. How do EVs contribute to vector development?
EVs are a natural, biocompatible delivery system that minimizes immune reactions while efficiently transferring genetic material, offering advantages over traditional vectors.
11. What are the leading manufacturing challenges for gene therapy vectors?
Manufacturing challenges include ensuring product consistency, maintaining high vector yields, minimizing contamination risks, and scaling up production while adhering to regulatory requirements.
12. What regulatory considerations impact gene therapy vector development?
Regulatory agencies require extensive safety, efficacy, and quality control testing. Developers must meet GMP standards, conduct thorough preclinical assessments, and follow clinical trial protocols to ensure vector safety and effectiveness.
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotech, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON CGT VECTORS
-
New AAV Reference Standards To Aid CQA Assessment
AAVs' complexity makes them difficult analytical testing subjects. The second part of a series describes USP's latest effort to help characterize the viral vectors.
-
New Reference Standards To Support Quality of AAV Raw And Starting Materials
The United States Pharmacopeia aims to help gene therapy manufacturers address the challenges that come with complex starting materials like endonuclease and plasmids.
-
How Do We Best Meet Future AAV Production Challenges?
In the recent past, viral vector manufacturers feared a capacity crunch. Now, with vastly improved productivity, predicting demand is a challenge.
-
The Latest Strategies For Quantifying AAV Capsid Titer
Let's take a look at the most commonly used methodologies, including established and emerging techniques, and evaluate the pros and cons of each.
-
Developing A Viral Gene Therapy Manufacturing Process
There is a growing popularity of adeno-associated virus (AAV) for delivering in-vivo gene therapies, a growing preference of transient transfection systems by biologics companies, and an increasing number of CDMOs offering this system as a platform process. This article describes a transient transfection AAV manufacturing process that uses a triple plasmid transfection strategy into HEK293T cells.
EDITORIAL PERSPECTIVES ON CGT VECTORS
-
Building A Universal AAV For Cancer
"Universal" isn't the first word most people think of when they think about AAVs. But what if there were such a thing? Siren Biotechnology's CEO, Dr. Nicole Paulk, shares the story behind developing the company's universal AAV immuno-gene therapy.
-
Improving AAV Purity Upstream With PCL Manufacturing
The downstream separation of AAV capsids affects end-product purity and has been identified as a key pain point of the industry. Ultragenyx’s Dennis Huang advises starting with higher-quality yields upstream.
-
Leveraging Directed Evolution To Optimize AAV Vector Design
4D Molecular Therapeutics (4DMT) CEO and co-founder, David Kirn, MD, talks about the company’s approach to vector optimization, its product development, and its stage-appropriate buildup of internal GMP manufacturing.
-
Moving Beyond AAV: The Next Generation Of Vectors In CGT
We catch up with Dr. Konstantin Konstantinov, CTO at Ring Therapeutics, and Ryan Crisman, Ph.D., cofounder and CTO at Umoja Biopharma, to get their thoughts on the future of new anellovirus vectors and existing lentiviral vector technology for in vivo gene delivery respectively.
-
Challenges Stunting The AAV Field
DeciBio conducted a survey among leaders in the AAV vector space to identify key pain points in the field. Dr. Carl Schoellhammer discusses these results and what they mean for the industry moving forward.
ON-DEMAND CGT VECTOR WEBINARS
-
Streamlining Viral Vector Development And Gene Therapy Manufacturing
Explore solutions for gene therapy production from experts, as they highlight the key features of a GMP manufacturing facility, including its technologies and infrastructure, through a virtual tour.
-
Using An Adherent Platform To Scale-Up An Upstream Viral Vector Process
Gain insight into a helper-dependent adenovirus (HDAd) process that covers everything from bench-scale and optimization to tech transfer.
-
Large-Scale LVV Production: Process Development To GMP Vector
Explore the key advantages of a proven approach to scale the lentiviral vector (LVV) production process (48L cf) in a bioreactor (200 L) without changing critical quality attributes (CQAs).
-
Optimization-By-Design — A Critical Factor In Viral Vector Scale Up
Learn how leveraging the expertise and capabilities of an experienced partner is a key factor in optimizing AAV processes using Design of Experiment (DoE), thereby reducing variability and risk.