Base Editing And Prime Editing: How They're Changing Gene Therapy

By Tyler Menichiello, Chief Editor, Bioprocess Online
It’s no question that CRISPR let the gen(i)e out of the bottle in terms of gene editing. It allowed scientists to specifically target genes in the genome easily and with high precision. Until recently, the only robust way to edit human cells was through nucleases (enzymes that cut or make breaks in DNA) like CRISPR-Cas9, which disrupts target gene sequences by making double-stranded breaks in DNA.
While nucleases like CRISPR-Cas9 can be effective for knocking out or disrupting a gene’s function, they can’t correct specific mutations in most cell types (i.e., most cells in a patient’s body). To treat most genetic diseases, genes need to be corrected, not disrupted. This where the work of Dr. David Liu comes in. Liu’s lab invented base editing, a highly efficient method of changing one DNA base into another, and prime editing, a newer but more versatile editing technology that can perform base edits as well as rewrites to parts of a gene. Beam Therapeutics and Prime Medicine were founded by Liu to bring base editing and prime editing to patients. To learn more about how these technologies are changing the gene therapy landscape, I spoke with Beam CEO, John Evans, and CSO, Dr. Gopi Shanker, as well as Prime’s CSO, Dr. Jeremy Duffield.
Editor’s note: Check out this episode of Cell & Gene: The Podcast where Dr. Peter Marks discusses base editing and prime editing and their potential to address unmet medical needs.

A Look At Base Editing And Prime Editing
At the heart of both technologies is the genome-targeting power of the CRISPR protein. Base editing leverages these targeting capabilities (without the double-strand breakage) and couples it with a deaminase to chemically change one DNA base into another. There are two major classes of base editors that, together, make four kinds of changes: cytosine to thymine, thymine to cytosine, adenine to guanine, and guanine to adenine. Shanker says this allows you to not only correct point mutations, but to limit, increase, or eliminate gene function altogether. As Evans puts it, “If you want a very highly efficient, precise, single genome modification, base editing is the winner.” It’s potent, predictable, and works in all cell types, meaning it can theoretically address a whole host of genetic diseases (potentially correcting an estimated 30% of known disease-causing mutations).

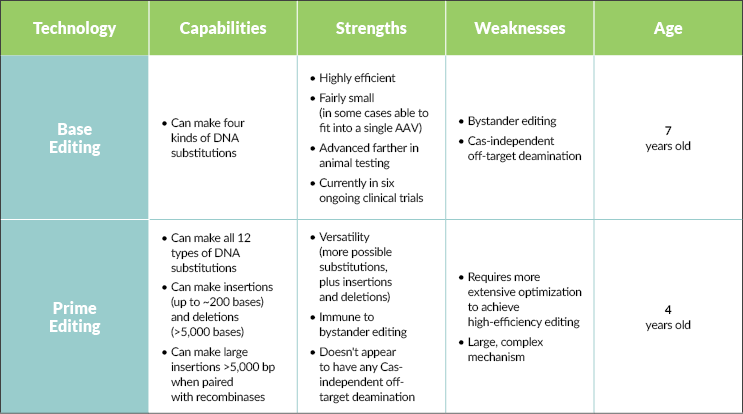

This highlights one of the challenges facing base editors — bystander editing (unwanted edits to bases near the target) and Cas-independent off-target deamination (changing bases not targeted by the CRISPR protein). Unlike base editors, prime editors are immune to bystander editing and don’t seem to exhibit any Cas-independent off-target deamination. They also seem to have very low off-target editing at any location in the genome that is not the target site.

For both technologies, delivery is still a hurdle. “Delivery is one key challenge that’s facing the entire field of genetic medicine,” Shanker says. Duffield echoes this sentiment. “Getting the editor enzyme into the nucleus of the right cells in the body remains a hard problem, and we’re all challenged by that.” Even though base editors are slightly smaller than prime editors and can fit inside an AAV capsid, targeting these AAVs to different tissues is challenging. “Base editing can work in any cell. If we’re able to get that editor to every cell we want to, then just think about the unmet needs we can address,” Shanker says.
Multiplex Editing
While base editing and prime editing have the potential to treat and even cure most monogenic diseases (those caused by single gene mutations), they also offer the exciting ability to combine or “stack” edits in a method known as multiplex editing. By coupling different guide RNAs, you can correct multiple mutations in a cell at once. This has implications for complex diseases with more than one causal mutation, as well as for engineering cell therapies.
“When we look at the future of cell therapy in general — making cells allogeneic, avoiding exhaustion, targeting solid tumors, navigating the tumor microenvironment, all these things —we’re pretty sure it’s going to take more edits than people are doing right now. And those higher levels of engineering, that’s going to bring you to base editing,” Evans says.
Beam has already seen some success in this approach with their Phase 1/2 cancer product, BEAM 201, “the first quad-edited cell in the industry.” According to Evans, it demonstrates the efficiency and potential of base editing to precisely engineer cells. “The reality is, we can do eight or more edits and not have any problem,” he says. This cannot be done effectively with other methods that rely on nucleases because they have a degree of unpredictability and offer less control compared to base editing or prime editing, especially as the number of edits adds up. “CRISPR technology can multiplex, but when it makes a lot of double-stranded breaks in the same cell, you can get these translocations of the whole chromosome coming across and knitting up with the wrong chromosome,” Duffield explains.
Multiplex editing can also eliminate the need for conditioning chemotherapy for transplant patients. Beam’s preclinical ESCAPE-1 platform and Prime Medicine’s Cimeio platform do this by combining a therapeutic edit (in the case of ESCAPE-1, for sickle cell disease) with an edit for a particular cell-surface antigen. This allows an antigen-specific antibody (administered after transplant) to “selectively deplete” the patient’s non-edited cells, ignoring the edited cells and allowing them to proliferate. “Not only are we helping patients cure their sickle cell disease, but we are also preventing them from undergoing chemo and all the horrible side effects that come with it,” Shanker explains.
Disrupting The Pharma Industry
Duffield bullishly tells me “prime editing is the technology that can fix all mutations in the human genome back to normal.” While the promises of base editing and prime editing are high, they don’t solve every problem facing the industry. The biggest limitation, of course, is the cost of cell and gene therapies. “We need to do more to bring down the costs of what we do to democratize this technology so we can truly bring it to patients around the world,” Evans says. Accomplishing this requires not only continued innovation, but cooperation with medical systems to establish payment plans and pricing models.
“I think it’s going to happen. All the right people are saying all the right things, but it’s going to be a transition. In the process, it’ll be quite disruptive to the pharmaceutical industry.” As we continue developing these technologies to cure diseases, less money will be spent treating them, and that will force the industry to evolve.
