Improving Patient Access To Allogeneic Cell Therapies
By Dr. Sanjay Srivastava, Managing Director, Cell & Gene Therapy Lead at Accenture; Martha Perez Linares, Management Consultant, Cell & Gene Therapies; and Alex Mora, Management Consultant, Cell & Gene Therapies at Accenture
Realizing the Promise of Allogeneic Cell Therapies
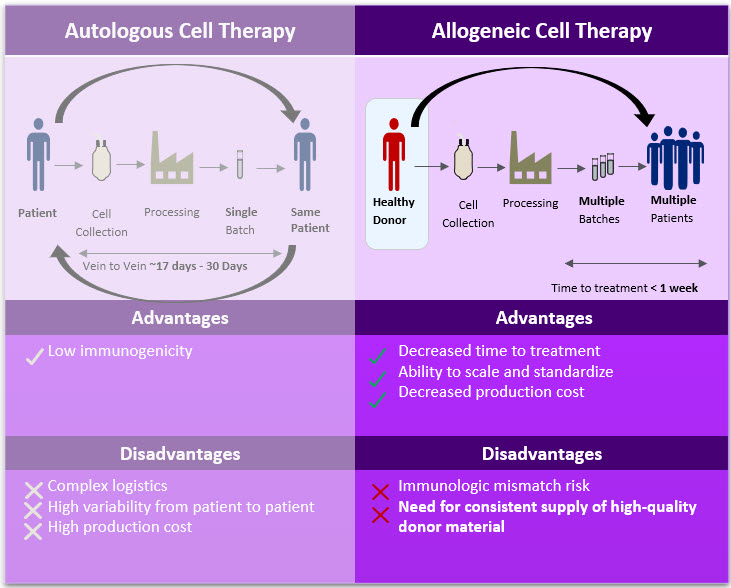
Figure 1. Autologous Cell Therapy vs. Allogeneic Cell Therapy Advantages & Disadvantages
Allogeneic cell therapies have the potential to broaden access to treatment for patients; however, building a reliable supply chain for cellular starting material is critical for success.
Allogeneic (allo) or “off-the-shelf” therapies promise greater patient accessibility to cell therapies through increased scalability, process and product standardization, simplified logistics, and lower production costs. This translates to shorter time to treatment and reduced costs for receiving treatment, as listed in Figure 1. Unlike autologous (auto) cell therapies, allo cell therapies rely on a single source of cellular material to produce a single batch of drug product that can treat multiple patients. To accomplish this, manufacturers leverage healthy donor cells that are processed according to the demands of a specific therapy.
While the benefits of allo cell therapies are numerous, building a reliable supply chain for cellular starting material is a critical first step. Consistent, high-quality starting material allows for predictable manufacturing and performance, but utilizing donor cells can also introduce safety risks to the patient (e.g., immunologic mismatch) and regulatory penalties if proper quality controls are not satisfied.
As allo cell therapy platforms continue to evolve, manufacturers face greater challenges to meet commercial demand. Developers must consider long-term solutions to develop their supply strategy early on to minimize risk and bottlenecks down the road. This article provides our views on:
- End-to-end donor management journey
- Challenges related to quality, supply chain and compliance
- Considerations to mitigate risk while sourcing cellular starting material
End-to-End Donor Management Journey
Starting with high quality donor material is critical for allogeneic cell therapy drug manufacturing, and this requires laying the groundwork for a high-touch, end-to-end donor management strategy.
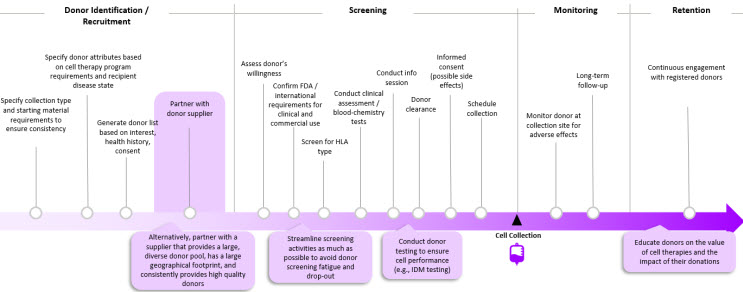
Figure 2. End-to-End Donor Management Journey
Planning an end-to-end donor management strategy (Figure 2) in the early stages of product development allows manufacturers to secure a reliable source of cellular starting material to establish a robust downstream supply chain at the start of manufacturing.
Once a manufacturer selects an allo therapy platform, the collection type and criteria need to be determined, including:
- Type of cells (e.g., pluripotent stem cells, cord blood, peripheral blood mononuclear cells)
- Volume or cell count
- Critical donor attributes (e.g., biomarkers)
Then, a list of potential donor profiles is generated based on these attributes, which also considers donors’ health history and willingness to donate cells.
There are several types of donor screenings that may need to be satisfied, including:
- Verbal Screening: Ensure that candidates are willing to donate cells
- Clinical Screening: Identify candidates with the right blood-chemistry characteristics and physiological characteristics; also conduct testing for viruses and infectious disease
- Immunologic Mismatch: Depending on the product, manufacturers may need to screen for human leukocyte antigen (HLA) type
Per regulatory requirements, donors must be educated on what to expect before, during, and after collection (e.g., potential side effects, intended use for cells) before consenting to donate their cells [1]. After the collection has taken place, the donor is monitored for adverse effects [2,3].
Just as it is important to constantly recruit new donors, it is also critical to retain a network of previously screened donors. Continuous donor engagement enables development of donor community. The latter can potentially serve as a source of donor advocates to generate “warm leads” of potential donors who can engage in company-specific donor recruitment campaigns.
Donor Management Considerations
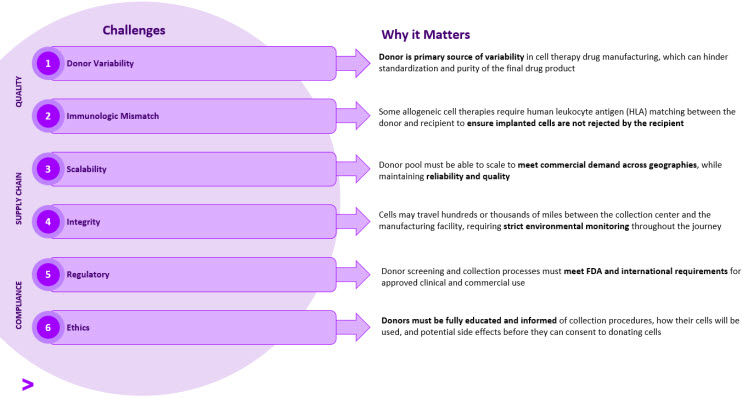
Figure 3. Allo Manufacturing & Supply Chain Challenges and Implications
From donor identification to manufacturing, quality, supply chain, and compliance should be top-of-mind for therapy developers.
Securing High Quality Cellular Starting Material
Donor variability is a major concern since it directly impacts clinical outcomes as well as the predictability of manufacturing operations. To maintain product standardization and purity throughout manufacturing, the starting material must be consistent across batches. In developing the cell collection procedures, manufacturers must determine the optimal collection time to maximize collection purity and yield. Downstream purification steps can be implemented in the manufacturing process (e.g., cell culture and cell expansion); however, it is important to note that these steps still rely on high quality starting material, since cell expansion steps will amplify any impurities. To avoid introducing other sources of impurities during manufacturing, it’s also important to develop a robust sourcing strategy for raw materials (e.g., reagents, vectors).
Some allo cell therapies require human leukocyte antigen (HLA) matching between the donor and recipient to ensure the implanted cells are not rejected by the recipient’s immune system. Genotype data and bioinformatics can be applied to facilitate immunogenetic selection for donor matching. Manufacturers can avoid the HLA-matching requirement all together by engineering PSC-derived cells to manipulate cells’ HLA-expression, thus avoiding rejection after transplantation [4].
To minimize variability between batches, manufacturers should consider using a master cell bank. Working with a donor supplier, manufacturers can establish a master cell bank with a single cell line, which can be differentiated into various working cell banks to manufacture multiple products.
Establishing a Robust Supply Chain
To secure a sustainable donor supply, manufacturers should start from a large donor registry and use broad selection criteria to avoid narrowing the donor pool too soon. Requirements related solely to gender, age, and blood type alone may decrease the available donor pool by at least 75%, and each additional screening requirement will shrink the donor pool even further.
In addition, manufacturers should establish their overall supply chain network capabilities (e.g., cryo shipment, temperature monitoring) and partners well in advance of commercialization. Manufacturers’ supply chain strategy must also include mitigation plans to ensure the cells stay viable if there are any delays in transferring the collected cells to the manufacturing site.
Adhering to Regulations and Ethical Standards
From a compliance perspective, manufacturers must ensure that donor screening, testing, and collection processes meet FDA and international requirements for approved clinical and commercial use. For instance, the Center for Biologics Evaluation and Research (CBER) and the European Medicines Agency (EMA) defined guidelines for physical examination, infectious disease marker (IDM) testing, and approved collection equipment [2,3]. Regional regulatory guidelines and directives apply to the location of the donors, manufacturing facilities, and patients, which introduces additional complexity if the donor material is sourced from a different country than the manufacturing facility.
Manufacturers must also adhere to ethical standards by ensuring that donors are fully educated and informed about the procedures they will undergo, how their cells will be used, and of any potential side effects from the collection [5]. Prior to proceeding with collection, manufacturers must obtain written consent from potential donors, which may include disclosure of options, voluntary choice, right of withdrawal, and consent at the time of donation [6]. Donors should not be compensated for their cells, since voluntary donations help ensure quality, safety, and compliance [7].
Donor Supply Strategy & Implementation Plan
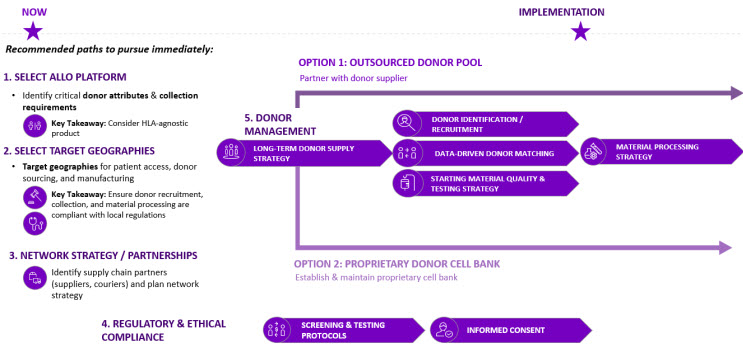
Figure 4. Donor Supply Implementation Plan
As allo cell therapy platforms continue to evolve and manufacturers move closer to commercialization, scaling donor pools to meet future demand will become a greater challenge. Developers must think about long-term solutions and supply strategies to secure consistent, high-quality material at scale early on during development. As outlined in Figure 4, there are five (5) key steps to defining the supply strategy:
- Define the vision and business strategy
- The supply strategy for an allo manufacturer will depend on the current state and desired future state of the business and platforms that they wish to develop. For example, a manufacturer who develops therapies using only PSC-derived cells may not require HLA-matching requirements, and their critical attributes will be significantly different than others. It is important for manufacturers to define their organization’s vision and desired platforms from the start to determine which capabilities they will need to develop.
- Select Target Geographies
- Manufacturers should identify target geographies for commercialization to develop strategies around patient access, cellular material sourcing, and manufacturing. Logistics and supplier partnerships may also vary by region.
- Network Strategy and Partnerships
- As the product moves through the development lifecycle, it is important to develop a network strategy for each geography. Manufacturers should build long lasting and strategic partnerships with vendors that are aligned with their business strategy and develop an operating model to collaboratively refine processes and share data.
- Understand Regulatory & Ethics Requirements
- Once the platforms and geographies are defined, it is a critical to understand the required controls to meet regulatory and ethics compliance across operations. Therefore, it is important to build a robust long-term supply strategy that complies with the market-specific regulations.
- Assess & Define the Long-Term Supply Strategy
- Considering all the risks and factors involved with each option during development is one of the most critical steps to seamlessly transition from clinical to commercial operations. Today, we have seen two main approaches to securing cellular starting material:
- Traditional E2E Donor Management Outsourcing
- Considering all the risks and factors involved with each option during development is one of the most critical steps to seamlessly transition from clinical to commercial operations. Today, we have seen two main approaches to securing cellular starting material:
The most common approach is establishing a partnership with a single source provider who offers end-to-end services including donor identification, recruitment, collection, and in some cases, even logistics and transportation. There are many established vendors in the current market landscape with robust donor management experience, resources, and broad networks to support.
However, with this approach, many manufacturers rely solely on dedicated donor pools, comprised of only a small subset of donors with the specific attributes required for the therapy. While this small subset of donors can meet clinical demand and support at a smaller scale, it is likely that these donors will experience life changes or will not be willing to donate continually for many years. Therefore, if a long-term partnership with a supplier is established to support commercial operations, it is critical to consider the supplier’s donor registry depth, breadth, and diversity to ensure constant replenishment of the pre-screened donor pool. Additionally, it is important to consider robust quality agreements early on, as there will be a high dependency on the supplier to successfully execute manufacturing operations.
- “Off-the-shelf” Proprietary Cell Bank
Some manufacturers are investing in “off-the-shelf” universal cell donor technology. With this novel approach, the intent is to avoid the donor matching process and minimize the immunity rejection risk by using a proprietary cell bank in a wide range of patients. This approach ultimately reduces time and costs in the overall production process and other partnership dependencies, and it provides a scalable solution. However, it is important to note that as of today, these technologies are still under development and have only been used for clinical purpose, and investment in developing in-house capabilities may result in higher costs.
When choosing either long-term supply approach, it is important to establish robust data infrastructure, including data lakes and advanced analytics capabilities, to collect and analyze data. This enables manufacturers to discern the impact of phenotype and genotype variables on clinical outcomes, and thus, maximize manufacturing standardization and optimization. Additionally, establishing robust material testing and preparation strategies is critical to achieve a consistent, high-quality final product.
Bottom Line
It is important to highlight that there is no “one size fits all” approach for allo cell therapy manufacturing. The starting material supply strategy during early development may vary significantly from the long-term commercial strategy; however, securing a reliable source of cellular starting material and establishing a robust downstream supply chain throughout the product lifecycle state will minimize manufacturing variability and reduce operational risks. Additionally, establishing a network strategy and identifying supply partners early on during development will allow manufacturers to develop robust agreements and capabilities to drive continuous improvement and optimization.
About the Authors
 Dr. Sanjay Srivastava – Managing Director, Cell & Gene Therapy Lead at Accenture
Dr. Sanjay Srivastava – Managing Director, Cell & Gene Therapy Lead at Accenture
Sanjay has a broad and deep expertise in the underpinning science and business challenges in developing and launching both in-vivo and ex-vivo cell & gene therapies. He directs his C> team at Accenture, who has global expertise in R&D, supply chain, manufacturing, and commercial operations.
 Martha Perez Linares – Management Consultant, Cell & Gene Therapies
Martha Perez Linares – Management Consultant, Cell & Gene Therapies
Martha is a Consultant in Accenture’s Life Sciences Supply Chain & Operations practice, with a specialized focus in Cell and Gene Therapies. Martha has over four years of biopharmaceutical industry and consulting experience working across multiple areas including Manufacturing, Quality Assurance and Operational Excellence.
 Alex Mora – Management Consultant, Cell & Gene Therapies
Alex Mora – Management Consultant, Cell & Gene Therapies
Alex is a Consultant in Accenture’s Life Sciences Commercial practice, with over four years of experience in Life Sciences business strategy and a specialized focus in Cell and Gene Therapies. She has extensive project experience working with both global pharma and biotech companies across various stages of the commercial journey, including execution of financial and capacity analyses, op model design, industry competitive scans, and roadmap development.
References
- Targeted recruitment of optimal donors for unrelated hematopoietic cell transplantation: The Stem Cell Club process
- Donor eligibility (screening and testing)
- EMA Directive 2004/23/EC
- Universal Donor Cells
- WHO guiding principles on human cell, tissue and organ transplantation
- Targeted recruitment of optimal donors for unrelated hematopoietic cell transplantation: The Stem Cell Club process
- Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells
