
ABOUT MILLIPORESIGMA
The development of gene therapies offers both remarkable opportunities and unprecedented challenges. The underlying drivers of many diseases can be addressed with this modality, offering new hope to patients. At the same time, the path to approval is complicated by a lack of robust process templates, an evolving regulatory landscape, and the urgency of unmet medical needs.
In this uncertain environment, where speed and quality are essential, working with an experienced partner will empower you to bring gene therapies to life.
MilliporeSigma, the life science business of Merck KgaA, Darmstadt, Germany, is an industry leader in the field of gene therapy with more than 30 years of experience and broad expertise across the entire workflow. We understand the unique pain points that innovators face, from optimizing upstream and downstream workflows to meeting accelerated manufacturing timelines and navigating new regulatory guidelines. We can proudly state that we were the first gene therapy CDMO to produce a commercial product following a successful regulatory inspection.
Our global organization is ready to address your specific needs with an integrated, customizable offering that encompasses:
- Industry-leading manufacturing technologies for upstream, downstream, and formulation
- Process development and scale-up services
- Viral vector contract development and manufacturing
- Analytical development and biosafety testing for cell and gene therapies
- Regulatory support
- Collaboration labs equipped with scientific experts, innovative technologies, and flexible non-GMP facilities
Rely on our extensive experience to overcome your process development, production, and testing challenges.
Visit our application webpages for more information on products and services for gene therapy development and manufacturing and discover how we’re partnering with innovators to shape the future of this exciting field.
WEBINARS AND PODCASTS
BROCHURES
- A Comprehensive Guide To Selecting The Right Prefilter
- A Viral Safety Solution Designed To Provide Retention Assurance And Productivity
- The Optimum Tangential Flow Filtration Devices For mAb's
- The Device Of Choice For Applications Requiring Higher Yield
- Regionalized Filter Supply Chain: Biopharma Supply, Reimagined
- Higher Titers In Small-Cell Culture Systems And Bench-Scale Bioreactors
- Achieve Higher Targeted Concentrations
- Innovation At Your Fingertips
- Understand Your Stability Programs
- AAV Gene Therapy: Charting The Course Through Clinical Challenges
- A High-Purity Excipient Suitable For High-Risk Vaccine Applications
- An Alternative Suitable For High-Risk Applications
- AAV Production: Your AAV CDMO Partner, Every Batch, Every Milestone
- Conical Tube Sampling Unit
- MGB:EDQ Probes For qPCR
- Robocolumn® And MiniChrom Chromatography Columns
- Viral Clearance Studies To Meet Your Timelines
- Viral Vector CTDMO Services
- Accelerating Adenovirus-Based Cell And Gene Therapy Manufacturing
- A Platform For Lentivirus-Based Cell And Gene Therapy Manufacturing
- Greener Detergents For Biopharmaceutical Applications
- Novel Enzyme For Nucleic Acid Removal At High Salt Concentrations
- Endonucleases With High Purity And Activity For Any Application
- Expert Partnership Contract Testing Services
- Innovative Single-Use Tangential Flow Filtration (TFF) Devices
- Filters Designed For Critical Small-Scale Gas Filtration
- Remote Control And Automation Software Optimized For Bioprocess
- Single-Use Sterile Sampling Assemblies
- M Lab™ Collaboration Centers
- Mobius® Essential Assemblies Specifications
- Ultrafiltration/Diafiltration (UF/DF) of Adeno-Associated Viruses (AAV)
- Next Generation Sequencing For Sequence Identity Confirmation And Variant Detection
- Viral Vector Manufacturing Capabilities
- Bio4C ProcessPad™ Software Datasheet
- Natrix® Q Chromatography Membrane Best Practices Guide
- EX-CELL® CD Insect Cell Medium
- Sf-RVN® Platform
- Sf-RVN® Insect Cell Line
- NovaSeptum® GO Sterile Sampling Systems Brochure
- Stemline® Platform Media – For Optimized Stem Cell Expansion
- CellPrime® rAlbumin
- Switching From Benzonase® Endonuclease Emprove® Expert To Benzonase® Endonuclease Safety Plus Emprove® Expert
- The VirusExpress™ Lentiviral Production Platform
- BioReliance® Gene Therapy Services
- Benzonase® endonuclease Safety Plus Emprove® Expert Data Sheet
- Benzonase® endonuclease Safety Plus Emprove® Expert Brochure
- Integrated Vector Production Capabilities
- Mobius® Power MIX 1000 And 2000 Single-Use Mixers
- Gene Therapy Capabilities For AAV And Lentivirus Production
- A Versatile And Modality-Agnostic Aseptic Autosampling Solution
PRODUCT GUIDES, DATA SHEETS & APPLICATION NOTES
- Accelerate LNP Manufacturing With Automated Process Development
- Application Of A Dual Spiking Strategy In Viral Clearance Studies
- Multi-Column Chromatography For Efficient Polishing Purification
- Virus Retention Performance Under Diverse Processing Conditions
- Supporting Complex Manufacturing For Liquid And Lyophilized Drug Products
- Cell Culture Media Filtration: Evaluating Cell Culture Performance
- Millipore Express® Family Guide
- A Viral Safety Solution Designed To Provide Retention Assurance And Productivity
- The Optimum Tangential Flow Filtration Devices For mAb's
- The Device Of Choice For Applications Requiring Higher Yield
VIDEOS
NEWS
- MilliporeSigma Introduces Comprehensive AAV Express Platform For Streamlined Gene Therapy Production
- MilliporeSigma Announces Siren Biotechnology As Winner Of Its North American Advance Biotech Grant
- MilliporeSigma Expands Its Optimized VirusExpress® Platform For Lentiviral Vectors, Further Enhancing Quality And De-Risking Cell And Gene Therapy Manufacturing
- MilliporeSigma Accelerates Readiness Of Bioprocessing Facility Of The Future
CONTACT INFORMATION
MilliporeSigma
400 Summit Drive
Burlington, MA 01803
UNITED STATES
Phone: 978-762-5100
FEATURED ARTICLES
-
Intensify your mAb manufacturing with optimized media, advanced filtration, and real-time analytics. Learn how integrated upstream and downstream solutions boost productivity and reduce costs.
-
Strategic, well-aligned partnerships between sponsors and CDMOs are key to efficient, scalable viral vector technology transfer.
-
Explore how sterility assurance, including sterilization validation and environmental monitoring, is essential for controlling microbial contamination in mammalian cell production to ensure patient safety.
-
Ensure consistency and quality in production with an advanced CCM fingerprinting technique that is capable of identifying over 100 media components to reduce variability and meet regulatory standards.
-
Ensuring media identity and consistency is vital in biologics manufacturing. Discover why quantifying cell culture media components before batch release helps overcome formulation complexity.
-
Explore the use of Next Generation Sequencing (NGS) to proactively screen raw materials in biopharmaceutical manufacturing, which aims to reduce viral contamination risks from animal-derived reagents.
-
Explore how molecular-based technologies, including PCR and Next Generation Sequencing, are transforming viral safety testing by offering broader, more effective alternatives to traditional animal-based methods like in vivo assays.
-
Navigating clinical trials for viral vector-based cell and gene therapies demands specialized expertise and regulatory insight. Learn how scientific acumen and strategic planning bring innovative therapies from lab to patient.
-
AAV-based gene therapies, while promising, require rigorous quality control through advanced methodologies to ensure safety, efficacy, and regulatory compliance.
-
The evolving field of viral vector production, driven by advances in gene and cell therapies, is facing increasing regulatory scrutiny and analytical demands.
-
Explore Cell & Gene therapy (CGT) products now within scope of the ICH Q5A guidance, technologies that can replace traditional testing strategies, viral clearance studies expected for vector products, and more.
-
Find out how to enhance CRISPR gene editing specificity by using Next Generation Sequencing to improve sgRNA quality control, addressing off-target effects and ensuring higher sequence fidelity.
-
Next Generation Sequencing (NGS) revolutionizes gene therapy by enhancing the development and safety of viral vectors like AAV and lentivirus. Discover more about its impact.
-
Gain insights into utilizing hybrid long-read and short-read sequencing to analyze adeno-associated virus capsids to enhance biosafety testing and genome assembly while achieving high identity mapping.
-
Discover how viral vector CDMO services can provide a solution to streamline gene therapy development, balancing clinical and commercial needs, to reduce delays and costs.
-
Explore how the eMERGE™ program digitizes the biopharma supply chain, streamlining raw material receipt, and enhancing efficiency with advanced shipping notifications and integrated systems.
-
21 CFR Part 11 and Annex 11 regulate electronic records and signatures in biomanufacturing, ensuring data integrity, security, and compliance while enabling efficient processes and regulatory alignment.
-
Continued process verification (CPV) ensures pharmaceutical processes remain controlled by monitoring key parameters, detecting variations, and maintaining product quality.
-
Many companies continue to manually collect data. Utilizing digital technologies can greatly improve productivity and efficiency by enabling real-time monitoring and streamlining regulatory reporting.
-
Scaling up viral vector production can be challenging, and ensuring consistent titers and activity requires careful optimization and technical expertise.
-
The complexity of manufacturing AAV products, coupled with the challenges linked to controlling their costs, underscore the importance of improving the efficiency and scalability of these processes.
-
RNA offers exciting potential for vaccines, gene therapies, and personalized medicine, but challenges related to stability, delivery, and manufacturing persist.
-
To fully realize the potential of mRNA as a therapeutic or vaccine modality, developers will need to employ cutting-edge analytical methods to ensure the safety and efficacy of these novel products.
-
Discover how implementing automated sampling systems enabled Takeda Pharmaceuticals to achieve new efficiencies in process development.
-
Learn about the processes and best practices applied at a GMP facility to optimize the production of working virus seed stocks and master virus seed stocks.
-
Examine how intensifying AAV production with high salt lysis and Benzonase® Salt Tolerant endonuclease enhances viral yield and infectivity while ensuring DNA removal for patient safety.
-
Explore the differences between research-use-only oligonucleotides and those manufactured in compliance with GMP 21 CFR 820.
-
Explore approaches for the capture and separation of capsids, upstream strategies for reducing the level of empty and partially filled capsids, trends in AAV capsid design impacting purification, and more.
-
When it comes to determining the appropriate analytics to inform early development, there are a number of variables to consider surrounding cost, operator expertise, and throughput.
-
The emerging trends in the biopharmaceutical industry are driving an even greater need for modular facilities and closed processing. Learn about the progression toward closed processing and more.
-
The COVID-19 pandemic underscored the vulnerability of global supply chains. Explore lessons learned from the pandemic, the concept of supply chain resilience, what to look for in a supplier, and more.
-
Gain insight into the recent advancements in connectivity and integration technology, the associated challenges, and the importance of a vendor-neutral approach.
-
We highlight strategies for ensuring the reliable delivery of health technologies with a focus on vaccines, mAbs, and new modalities manufactured by biotechnology companies and institutes.
-
The analysis of over 10 variant callers and other bioinformatics tools for viral variant detection are discussed to better understand how the outcomes can improve gene therapy product characterization.
-
Explore key points from the presentation MilliporeSigma delivered on the analysis and comparison of manufacturing costs between traditional and modern vaccines at the World Vaccine Congress Washington 2023.
-
Nearly forty biopharmaceutical companies, CDMOs, and research institute executives took part in interviews on the future of vaccine manufacturing. Explore the key findings following these interviews.
-
Our aim was to create scalable, single-use sparger and impeller designs that offer maximum performance capable of supporting high viable cell densities while cognizant of cell shear sensitivities.
-
The aim of this work was to use a power number model-based approach for the design of a scalable single-use impeller capable of reaching high power densities with low mixing time and tip speed.
-
Discover an experienced, flexible, and capable partner that can help you optimize production from MCB and WCB manufacturing to safe cell bank storage.
-
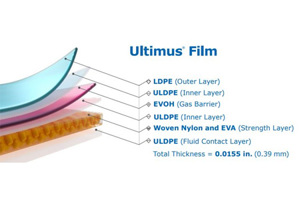
In the biopharmaceutical industry, bag leaks remain a top pain point for customers. Ultimus® film addresses this costly issue, offering superior protection against leaks, abrasions, tears, and material fatigue.
-
Following successful Industry 4.0 implementation in the automotive, communications, aerospace, and other industries, the biopharma industry is now joining the digital revolution.
-
A growing number of AAV product developers are recognizing the value in moving to suspension as early in development as possible in order to optimize production.
-
Since production templates depend on cell culture processes, biopharmaceuticals are susceptible to adventitious agent contamination. Explore a holistic overview of viral safety consolidating decades of expertise and process understanding.
-
Learn how we simplify bringing life-changing molecules to market, from pre-clinical to commercial, with our CTDMO services designed to support global clients.
-
MilliporeSigma experts and biomanufacturers discuss the importance of integration, collaboration, and education to address the industry paradigm shift towards novel modalities, continuous bioprocessing, and better risk mitigation.
-
Guidance for your plasmid DNA downstream process development, exploring cell harvest, lysis, neutralization and clarification; chromatographic purification; TFF; and sterile filtration unit operations.
-
With a diverse landscape of potential partners, it is important to know what qualities to look for in a technology provider and how to traverse the challenges inherent in managing multiple relationships.
-
Because viral safety is essential in the manufacture of biopharmaceuticals, we developed a proven Sf9-rhabdovirus-negative insect cell line that improves the safety profile of our customers’ bioprocesses.
-
It is critical to vet not just the expertise available at a CDMO, but also the effectiveness of their facility design to reduce risk and meet scale-up needs.
-
The goal of clarification is to prepare the cell culture feed stream for downstream chromatography and purification. In this poster, we explore data driven strategies for clarifying harvest feed streams.