Integrated Vector Production Capabilities
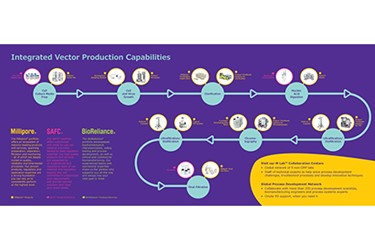
Partnering Together for Faster Vector Production and Testing
BioReliance® Viral and Gene Therapy Manufacturing
World-class process development and manufacturing for viral therapeutic products. From small-scale toxicology and Phase I material to commercial-scale manufacturing and fill finish, our flexible facilities and expert staff are leaders in viral product manufacturing.
BioReliance® Biosafety Testing Services
For viral gene therapy, BioReliance® biosafety testing services provide a comprehensive range of assays and services to support every stage of your biologics development and manufacturing process. From cell banking and cell line characterization, through lot release testing and product characterization, GMP testing is offered with leading turn-around times and scientific expertise.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
