ABOUT CORNING LIFE SCIENCES
Corning Life Sciences is a global, leading manufacturer of lab tools for growing cells, bioprocess manufacturing, liquid handling, benchtop equipment, among other solutions for life sciences. Corning strives to improve efficiencies and develop innovations that enable researchers to harness the power of cells to create breakthrough discoveries in research areas like cancer, primary cells, stem cells, drug discovery, cell and gene therapy and lab automation. Learn more at www.corning.com/lifesciences.
FEATURED ARTICLES
-
Transitioning to closed systems enables the 100-fold expansion of engineered cells. Achieve high yields and maintain anti-cancer potency while reducing contamination risks and labor.
-
Discover scalable strategies for high-yield adherent cell culture. Learn how gas-permeable technology optimizes MSC expansion, viral vector production, and extracellular vesicle secretion.
-
Effective cell concentration and buffer exchange are crucial in MSC biomanufacturing for safety and efficacy. Compare the trade-offs of centrifugation, filtration, and elutriation to optimize your process.
-
Learn about the defining criteria and varied tissue sources of MSCs. Discover their expanding role in therapies targeting inflammatory conditions and tissue regeneration.
-
Achieving over 870 million viable, quality mesenchymal stem/stromal cells is possible. This efficient cell culture method scales bone marrow-derived MSCs for clinical needs.
-
Maximize mesenchymal stem cell (MSC) expansion with a simplified culture process. New data demonstrates a high-performance, xeno-free media that eliminates the need for coatings or media exchanges.
-
Optimize your lab's workflow by understanding the trade-offs between rapid enzymatic isolation and the superior cell integrity of explant culture for mesenchymal stem cells.
-
Learn how innovative cell culture vessel design enables high-density cell growth with superior gas exchange and a closed system to scale up bioproduction efficiently while maintaining batch-to-batch consistency.
-
Mesenchymal stem cells (MSCs) show great promise in regenerative medicine, but scaling their production for clinical use is a key challenge that requires careful optimization and planning.
-
Explore the journey of producing MSC-derived cell therapies, from initial isolation and expansion to final product formulation and storage, and learn how to optimize each step.
VIDEOS
-
Traditional nanoparticle analysis creates process bottlenecks. Learn about an interferometric light microscopy method that provides accurate physical titer and size distribution from raw samples in under a minute.
-
Learn about advancing cell therapy manufacturing through scalable and efficient mesenchymal stem cell (MSC) production. Discover how to improve cell yield and streamline your bioprocess.
-
The Corning CellCube system enables large-scale adherent cell culture with uniform laminar flow, optimizing growth. Its closed system design ensures aseptic scalability with AseptiQuick® connectors.
-
Corning offers standard and custom cell culture media, vessels, and surfaces to support all growth stages. A vertically integrated supply chain ensures reliability and quality.
-
Ensure uninterrupted cell culture success with reliable, high-quality animal-derived serum, including FBS, with consistent performance. Learn how to secure your supply.
-
As part of our complete cell culture offering, Corning’s comprehensive line of standard and custom cell culture media helps create an optimal environment for all stages of cell culture growth and scaling.
-
Corning Closed System solutions for CellSTACK vessels streamline the cell culture process while reducing the risk of contamination.
-
During each Cell & Gene Live, our audience can submit real-time questions for our expert panelists. Watch and listen as Michael Blackton and John Lee provide detailed, insightful responses to our audience questions on everything from how changing from a lab-based adherent protocol to suspension scalable protocol improved the cell product to whether a container with lesser cells, identical cell density, and composition be used for establishing stability for cell therapy products, and much more.
-
During Cell & Gene Live, "Scaling Cell Therapies Part 2: Establishing Comparability in Manufacturing," Michael Blackton and John Lee provided detail on how successful comparability studies are designed and executed. They covered the in-process and release testing requirements, as well as the common and uncommon pit falls that have been encountered.
-
For cell-based products, donor-to-donor variability is the primary source of complexity associated with establishing product comparability. In this segment of Cell & Gene Live, "Scaling Cell Therapies Part 2: Establishing Comparability in Manufacturing," Michael Blackton and John Lee cover why donor-to-donor variability is an on-going challenge and potential solutions.
-
Here, during this segment of Cell & Gene Live, "Scaling Cell Therapies Part 2: Establishing Comparability in Manufacturing," Michael Blackton and John Lee highlight the various ways to demonstrate our understanding of how the process influences the product, as well as how the product’s structure influences its clinical function.
-
As a therapy proceeds through Phases I / II and into Phase III clinical trials, the comparability burden increases. As the body of knowledge increases, the comparability burden evolves. Some tests may be removed while others are added, and assumptions may change with data. In this segment of Cell & Gene Live, "Scaling Cell Therapies Part 2: Establishing Comparability in Manufacturing," Michael Blackton and John Lee break down the comparability requirements from early- to late-stage programs.
-
During this Cell & Gene Live, "Scaling Cell Therapies Part 2: Establishing Comparability in Manufacturing," Cell & Gene's Chief Editor, Erin Harris, Michael Blackton, former SVP Cell Therapy Manufacturing at Nurix Therapeutics, and John Lee, SVP, Head of Cell Therapy at Center for Breakthrough Medicines, talk through the CGT sector's greatest struggles with comparability. Indeed in this segment, Blackton and Lee cover the sector's biggest challenges to date from the need to truly understand the product to precise analytics and eliminating silos, and more.
-
In this video our application scientists walk you through common research goals and the most suitable 3D cell culture solutions for each.
-
There are many harvesting options depending on the cell line and application. In this video we discuss some tips and tricks for harvesting cells.
-
The Corning® Matribot® bioprinter uses a revolutionary cooling syringe printhead technology that allows you to dispense 3D droplets or droplet arrays for organoid applications.
-
With gas-permeable film, this closed system offers more surface area within a compact footprint. Watch this short video to learn more.
-
We visit the MEARY Center to see how they produce new cells and new gene therapy drugs to treat patients suffering from autoimmune, hematological, and cardiac diseases, and so much more.
-
In the complex world of cell and gene therapy, having a seasoned partner to guide you through uncharted production territory is critical to success.
-
Corning’s Field Applications Specialists work with scientists like you to overcome complex manufacturing challenges and streamline the path to production.
-
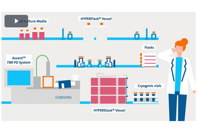
The Ascent FBR System’s novel mesh substrate intensifies cell growth surface area to produce high cell yields more efficiently.
-
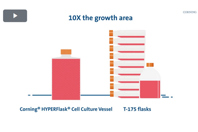
When you need a reliable path from research to bioprocessing try efficient tools like the Corning® HYPERFlask® cell culture vessel.
CONTACT INFORMATION
Corning Life Sciences
836 North Street
Tewksbury, MA 01876
UNITED STATES
Phone: 978-442-2288
Contact: Nicole Athanas
PRODUCTS
BROCHURES
- MSCulture Max™ Media Frequently Asked Questions
- MSCulture Max™ Media
- Ascent® Fixed Bed Reactor System 5
- HYPERStack® Cell Culture Vessel
- CellSTACK® Culture Chambers
- Rapidly Create Closed System Assemblies For A Customized Configuration
- Corning® CellCube® System
- Closed System Solutions
- Ascent® Fixed Bed Reactor System 100
- Ascent® Fixed Bed Reactor System
- High Quality Water: Cell Culture Grade, Molecular Biology Grade, Reagent Grade
- Exploring A Modular, Scalable Platform For Adherent Cell Culture
- High Quality Water: Reliable Purity For Critical Applications
- Corning® Erlenmeyer Flasks: Versatile Vessels For Suspension Cell Culture, Seed Train, Closed Processing, Storage, And Other Applications
- Measure The Size And Concentration Of Nanoparticles In One Drop
- Frequently Asked Questions: Corning® CellCube® System
- Film Types For Corning® Single-Use Containers
- Automate To Accelerate: Meet The Corning® Matribot® Bioprinter
- Viral Vector Manufacturing And Cell Harvesting From A Single Bioreactor?
- Stack Facts: Scaling Up From Research To Clinical Applications
- Corning® Fetal Bovine Serum
- Corning® CellSTACK® Culture Chamber PEIpro® Transfection Protocol
- Guidelines For Use Of Erlenmeyer Flasks
- Corning® 5L Erlenmeyer Aseptic Transfer Cap For Transfer By Vacuum Or Positive Pressure
- Corning® CellCube® Culture System Cell Expansion