
WEBINARS
Scalable Production Of Lentivirus For Research To Commercial Manufacturing
Join our webinar on scaling the CTS LV-MAX System in bioreactors, covering transfection scalability, Thermo S.U.B. advantages, and DynaSpin’s efficient gene therapy integration.

An Innovative Approach To High Aggregate Challenges
In this webinar, hear from a technical specialist on addressing the challenges with antibody aggregates and process related impurity removal using a new high performance chromatography resins

Interview With Pharma Analytics Field Application Specialist - Sandi True
Hear Sandi True, a Field Applications Specialist discuss helping customers navigate challenges often encountered when evaluating and implementing Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.

Supply Resiliency For Bioprocessing Amid Global Volatility
Build bioprocessing resiliency with strategic supply chain management. Learn to meet quality, compliance, and time-to-market demands amid global volatility with effective sourcing.

Enhancing AAV Purification In Gene Therapy Manufacturing
Review chromatography solutions for purifying multiple AAV serotypes, with specialists’ insights on bioprocessing and downstream optimization.

Multi-Omics And Bioinformatics In Cell Culture Media Design
Learn about the impact of a multi-omics analysis, utilizing proteomics and metabolomics, in the development and optimization of media with the goal of improving titer, and making development and manufacturing more efficient.

Simple In-House Mycoplasma Testing Method For Regulatory Expectations And Rapid, Confident, And Actionable Results
In this webinar, we present an overview of worldwide regulatory guidance for mycoplasma testing, as well as a mycoplasma testing method that has been accepted by regulatory authorities worldwide across various therapeutic modalities.
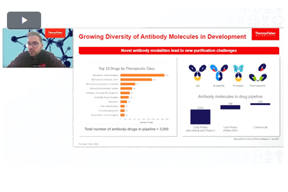
Tools For Efficient Downstream Processing Of Antibody-Based Therapeutics
Watch and discover how to effectively utilize chromatography tools for improved purification of therapeutic monoclonal antibodies and antibody derivatives.
Scalable Purification Of In Vitro Transcribed mRNA
Learn more about how affinity-based mRNA chromatography resin facilitates the purification and isolation of mRNA from in vitro transcription (IVT) manufacturing processes.

Rapid Micro Methods In QC Micro Testing: A NIBRT Perspective
Learn about some of the advanced Rapid Micro Method systems that are being adopted by biopharma companies worldwide, and how they are changing the face of QC Microbiological testing.

Innovative Contamination Control: Enabling Integrity And Efficiency
An integrated approach can enhance contamination control, boost process efficiency, and ensure the production of high-quality cell therapy products.

Manufacturing Considerations For Viral & Non-Viral Platform Selection
In this webinar, a panel of experts spanning the lentiviral vector, exosome and oncolytic virus fields will discuss the impact of manufacturing considerations on their respective platform selection and ongoing product/process development strategies.

Interview With Nico Chow And Sandi True, Field Applications Specialists
Hear Nico Chow and Sandi True, Field Applications Specialists, discuss working with customers to help them evaluate and implement Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.
Supporting Development Of mRNA Therapeutics By Enabling Commercial-Scale mRNA Purification
Discover the next generation of scalable affinity capture for high-quality mRNA purification. Learn how a simple, reliable binding mechanism can maximize efficiency and simplify your manufacturing process.

The Role of Peptones In Enhancing Efficiency
Discover how peptones enhance vaccine manufacturing efficiency with valuable insights into cost-effective solutions, variability control in biologically derived materials, and more.

Basic Cell Culture Education Series: CHO Media
This Knowledge Culture Basic Education Series on CHO media will explore CHO cells: their lineage, historical culture media, and process improvements. We will dive into how to optimize cell culture performance for superior outcomes.

Successful Microbial Testing And Identification
In this presentation, one of our industry-leading customers discusses how they've successfully implemented the Applied Biosystems MicroSEQ Microbial Identification System in their laboratory testing.

Knowledge Culture Basics — Gene Therapy Education Series: Lentiviral Vectors
In this session of our ongoing education series on lentiviral vectors, we delve further into principles of lentiviral transduction and challenges in suspension-based lentivirus production.

Analytical Solutions To Help Accelerate Bioprocessing Success
Unlock the power of data-driven bioprocessing. Discover how advanced analytics can optimize your cell culture process, improve performance, and accelerate success.

Speed Up Process Development With HTS Tools For Affinity Chromatography
High Throughput Screening (HTS) chromatography tools can accelerate process development and reduce material usage. Learn about a solution that addresses challenges with their use in new modalities.
Building A Purification Toolkit For An Expanding Variety Of mAb Therapeutics
Explore techniques for optimizing purification toolkits for various monoclonal antibody formats. This session covers resin and buffer selection for efficient downstream process development.

Practical Solutions For Protein Analytics And Residual DNA Testing
Discover practical solutions for common quality control bottlenecks in biotherapy manufacturing. Learn how to improve protein analytics and DNA testing robustness and accelerate time to market.

Innovative Strategies For Residual DNA And Viral Titre Quantitation
Discover how digital PCR improves the accuracy of residual host cell DNA and viral titre quantification in biotherapeutic manufacturing to enhance product quality, safety, and regulatory compliance.

A Straightforward Path Toward Regulatory Compliance, Data Integrity, And Computer Systems Validation
Learn practical strategies for validating microbial identification systems in cGMP. Understand regulatory guidance and master a complete Computer Systems Validation plan for full compliance.
Optimization Of Anion Exchange Purification For The Large-Scale Production Of Plasmid DNA
In this webinar, we discuss pDNA purification, including how to identify optimal conditions for various chromatography media and optimize recovery and impurity removal.

CHO Solutions Day Part 1
This CHO Solutions Day presentation offers valuable insights into new media and feed systems, a successful scale-up story, and how multi-omics analysis can enhance your cell culture.
Smart Media Screening And Design: Saving Time And Boosting Titers
Optimizing your cell culture media selection is crucial for clinical advancement. Learn how high throughput screening techniques accelerate media and feed optimization to significantly boost your biologics' titers.

Driving Sustainability In Bioprocessing With Biobased Single-Use Solutions
Discover how biobased materials in bioprocessing enable measurable carbon emission reductions. Learn practical strategies to meet decarbonization goals and adopt greener, operationally sound practices.
Enhancing Protein Quality Through Optimized Galactosylation
Advance your understanding of critical protein engineering. Explore expert strategies for optimizing complex molecular structures, paving the way for more effective and consistent biological products.
Efficient Downstream Processing Of Antibody-Based Therapeutics – A CDMO Perspective
Discover efficient downstream processing strategies for antibody-based therapeutics. Explore key considerations and trade-offs in purity, yield, speed, and cost, from a CDMO perspective.
