ABOUT ROOSTERBIO
RoosterBio accelerates human mesenchymal stem/stromal cell (hMSC) and extracellular vesicle (EV) product and process development to fuel the rapid commercialization of scalable regenerative cures. Our high-quality hMSCs, bioprocess media, genetic engineering tools, and EV production solutions are paired with expert bioprocessing knowledge to progress therapeutic developers from concept to first-in-human testing and commercial manufacturing at reduced cost and increased productivity. With optimized, scalable processes, Type 2 Drug Master Files, and cGMP products, we have enabled therapeutic programs to traverse their path to clinical translation in under 1 year.
RoosterBio is driven by our client's success and creating a world where safe and effective regenerative medicines are rapidly developed and widely available on a global scale.
FEATURED ARTICLES
-
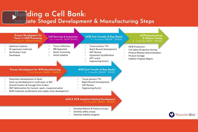
Discover how effective cell banking and scale-up strategies can reduce risk and cost in advanced therapy manufacturing. Learn practical ways to meet increasing cell and exosome quantity needs.
-
Uncover how analytical tools assess the impact of production process choices and tissue sources on the quality of cell-derived extracellular vesicles (EVs).
-
Biological components in conditioned media are a consistent problem during downstream processing of EVs. A novel in-process reagent prevents these issues and improves particle recovery 10-fold while maintaining CQAs.
-
EV production scale increases require paired robust, scalable, and cost-effective downstream processes. Process parameters can be tailored for therapeutic exosome programs and allow for maximum exosome recovery.
-
A new chemically defined fed-batch bioprocess medium simplifies hMSC-EV production, increasing yields by 2-4x while maintaining EV quality across donors and tissue types in scalable GMP-compatible environments.
-
One of the challenges faced in EV production is scaling up manufacturing processes. Discover a reproducible and scalable microcarrier-based process for EV production in a stirred tank bioreactor system.
-
EV production must align with CMC and regulatory standards before it can reach its full potential. This study compares conditioned medium across scalable platforms to optimize EV manufacturing for the clinic.
-
From discovery to patient doses, viral vectors and exosomes face a complex journey. Researchers must navigate five key downstream processing challenges to ensure these therapies reach their full potential.
-
Like a referee in a contest, advanced analytical methods are essential to mediate the push and pull of different factors influencing therapeutic efficacy.
-
Learn about a novel solution dramatically improving the purification and yield of promising nanoscale vesicles for medical applications.
-
Explore how synthetic biology and a "scale-out" biomanufacturing strategy can unlock "infinite diversity in infinite combinations," optimizing MSC functionality for superior outcomes.
-
CD73, found in MSC exosomes, is a key player in tissue repair. Discover how this protein contributes to cell survival and the intricate process of wound healing.
-
For efficient, scalable, and cost-effective cell and exosome production, 3D cell culture is the answer. Learn how this advanced approach surpasses 2D limitations, unlocking new potential for therapies.
-
Biomanufacturing leaders unite to optimize exosome/EV production. Explore their combined upstream/downstream approach for optimized cell culture, purification, and quality control.
-
Mesenchymal Stem Cells (MSCs) hold immense therapeutic promise. Can we harness their potential by making them more like consistent cell lines, without losing their unique advantages? Let's explore.
-
Researchers are exploring extracellular vesicles to deliver CARs, potentially offering safer, more accessible, and effective treatments compared to current CAR-T cell therapies.
-
Modern solutions are continuing to be developed to help those working in biologics overcome historic Exosomes / Extracellular Vesicles challenges.
-
While more biotech companies are entering the market, advanced therapy startups can still benefit greatly from product and service options that help address challenges in the MSC or EV / exosome space.
-
Take a look at important information and data from PubMed publications regarding cancer and fibrosis research and as well as learning about MSCs & their EVs/Exosomes.
-
Gain insight into the importance of scalability for future clinical products made from extracellular vesicles, the quality of peer-reviewed extracellular vesicle/exosome science, and more.
CONTACT INFORMATION
RoosterBio
5295 Westview Drive Suite 275
Frederick, MD 21703
UNITED STATES
Phone: 240-831-4914
RESOURCES
-
When MSCs are genome-edited, it will inevitably be done with cell therapy in mind and should involve complementary breakthroughs to scale up and industrialize gene delivery.
-
Available in multiple tissues origins and from multiple donors, RoosterBio’s human mesenchymal stem/stromal cells (hMSCs) are sourced under strict guidelines, manufactured with highly standardized processes, and supported by 1st in class characterization.
-
Eliminate media exchanges with RoosterNourish™ hMSC expansion medium and RoosterReplenish™ in-process bioreactor feed.
-
Standardize the production of exosomes/extracellular vesicles with a translation-friendly, scalable system designed to streamline your process from cell-expansion to optimized EV collection yields.
-
RoosterGEM™ is a complete genetic engineering medium that allows researchers and product developers to confidently engineer their therapeutic product with simplified end-to-end processes.
-
RoosterBio’s Cell & Media Kits allow you to economically screen multiple hMSC donors or tissues and rapidly evaluate RoosterBio’s media exchange-free system.
-
RoosterBio’s CliniControl™ products include human mesenchymal stem/stromal cells, bioprocess expansion media, and exosome/extracellular vesicle production media manufactured in compliance with cGMPs and supported by U.S. FDA Type II Master Files.
-
The path to IND approval for cell-based therapies can be challenging and time-consuming with strict regulatory requirements.
-
Advanced therapy programs are complex arrays of upstream and downstream processes that must reliably work together to produce consistent products worthy of clinical use.
BROCHURES
- Ready-To-Use Exosomes That Streamline R&D
- RoosterGEM™ - A Complete Genetic Engineering Medium
- RoosterVial™ MSCs
- RoosterNourish™ Mesenchymal Stromal Cell (MSC) Expansion Media
- RoosterCollect™ Exosome/Extracellular Vesicle Production
- RoosterReplenish™ hMSC Bioreactor Feed
- RoosterCollect EV Pro™ - Extracellular Vesicle (EV) Production
- RoosterBio Process Development Services