ABOUT SINGLE USE SUPPORT
Single Use Support is a solution provider of innovative single use technologies for fluid and cold chain management process solutions. At Single Use Support, we have set ourselves the goal to provide the biopharmaceutical industry with solutions that support them in increasing the reliability of storage and shipping and in turn minimizing the loss of valuable high-quality drug substance. On the basis of single use bags, Single Use Support offers biopharma companies access to a new and 100% secure logistics process for liquids.
The innovative process solution provider enables its customers to set up highly robust bulk drug substance freeze/transportation/storage processes for single-use bags, which in turn in creases patient safety and reduces the risk of bio-contamination and product loss. With the utilization of Single Use Support technology, bigger volumes of high-quality substance can be shipped internationally in a faster and absolutely secure manner, and at a lower loading volume.
Single Use Support was founded in 2016 and is based in Kufstein, Austria. At the global headquarters, new products are developed in cooperation with international pharmaceutical companies.
In the past years, Single Use Support GmbH has developed technologies that bridge the gap in handling of biopharmaceuticals. Its entire end-to-end process for biopharma liquid logistics are applicable to various areas, such as bulk drug substance, cell & gene therapies and seed train intensification. The core product RoSS, a single use bag protective shell system developed by Single Use Support, provides the basis for technologies and services around filling, freezing, shipping, storing, thawing and draining regardless of the biopharmaceutical product and the primary packaging used.
CONTACT INFORMATION
Single Use Support
Endach 36
Kufstein, 6330
AUSTRIA
Contact: Thomas Wurm
FEATURED ARTICLES
-
Learn how the transition to plate-based freezing with single-use technologies improves scalability and contamination control while ensuring uniform thermal transfer for high-value bulk drug substances.
-
Until recently, only isolated solutions for working filling, freezing/thawing and transporting sensitive drug materials were available. Learn how we've addressed this problem with end-to-end bottle solutions.
-
Uneven ice formation during bottle freezing creates a "Volcano Effect," pushing solutes into highly concentrated zones. This test-based study explains this risk to drug substance quality.
-
Learn five critical considerations for freezing bottles, including uniform rate control, container integrity, and selecting the right freezing technology to maintain product stability and process efficiency.
-
Manual bottle filling can introduce significant risks, impacting accuracy, sterility, and compliance. Learn how automation can improve product quality, enhance patient safety, and increase efficiency.
-
Explore the dynamic field of therapeutic biotechnology, examining the intricacies of mAb manufacturing and uncovering the often-overlooked factors driving high production costs.
-
Explore best practices for cryopreserving small-volume advanced therapy medicinal products (ATMPs), emphasizing sterility, cell viability, and efficiency using innovative single-use systems and aseptic connectors.
-
Tired of manual manipulation risks in biopharmaceuticals? Discover how automated homogenization ensures consistent cell counts and concentration levels, improving patient safety and process reproducibility.
-
Dual sourcing strengthens the pharmaceutical supply chain for single-use assemblies. Learn how this strategy and multi-sourcing can enhance resilience and adaptability in the face of disruptions.
-
Take a closer look into research aimed at enhancing the cryogenic freezing process of the mammalian CHO-K1 cell line using a controlled-rate liquid nitrogen freezer.
-
Explore the challenges of adding cryoprotectants, how they can help reduce the effects of harmful conditions like ice crystals, and the need for future stability studies and substitutes for toxic CPAs.
-
Here we explore the use of viral vectors in gene therapies, as well as factors that negatively affect them during manufacturing.
-
See how adopting the standardized and fully automated processes of Industry 4.0 can contribute to your cost efficiency, patient safety, and sustainability in pharmaceutical manufacturing.
-
Single-use technology is playing a large role in increasing flexibility and reducing contamination risks for the biopharmaceutical industry. Take a deeper look into all of the advantages and challenges.
-
In order to advance viral vectors like AAVs, there's a growing need for technologies and platforms that facilitate reliable and safe handling all the way from production to shipping and storage.
-
Take a closer look at the process of cryopreservation, which role it plays for cell banking, and which challenges have to be overcome to reach the successful cryopreservation of cells.
-
Review important factors in cell viability and cell health and how this information is crucial to researchers and cGMP cell banking.
-
Learn about the crucial role vectors play in delivering genes, types of non-viral vectors, the challenges with non-viral vectors in gene therapy, and the advantages of lipid-based nanoparticles.
-
Delve into the medical applications of lipid nanoparticles with a focus on their role in targeted drug delivery, particularly in cancer treatment, gene therapy, and vaccine development.
-
Explore advanced fluid management for cell culture freezing, cell banking and cell therapy.
-
Learn more about the application of ADCs, the challenges of manufacturing them, and tools for handling them in closed systems without exposing them to the environment.
-
Learn more about the challenge of controlling cryogenic freezing, especially with liquid nitrogen, and some innovative solutions being presented.
-
Learn about a high performing bulk filtration, filling and draining platform for primary packages such as single use bags, bottles and vials.
-
Explore viral vectors, an indispensable means to modify the genetic material of human cells, and their future in gene therapy.
-
Explore ways to tackle the process step of freezing and thawing when it comes to manufacturing quality biopharmaceutical products, like ADCs, at scale.
-
Learn what makes controlled cryogenic freezing such a vital step in the cold chain of the majority of biologics and understand more about different ranges of temperatures and freezing performance.
-
Join the discussion on how a new proprietary cryoprotectant technology and our a plate-based freeze/thaw unit can be uniquely combined to prepare Cryopreserved Thaw and Use Cell Plates.
-
Learn how fluid management in biopharma has changed considerably in recent years, and what we do to stay ahead.
-
Read how single-use technologies and the modular design options they offer can meet the requirements of both laboratory- and bulk-scale production without the need for major adaptations.
-
Read how single-use technologies are making supply chains more efficient and reduce product loss by providing specific protective technologies, equipped with Pharma 4.0 mechanisms.
-
We review the pre-use post sterilization integrity testing process and how its compatibility with single-use technology offers advantages compared to stainless-steel systems.
-
Read how the implementation of Pharma 4.0 standards and SOPs aims to reduce the different types of human error through the automation of processes that have traditionally involved human performance.
-
These insights provide a brief primer on Pharma 4.0 and will convey the key principles of the digital revolution in the pharma sector.
-
This article will give an overview of the inline buffer dilution process and discuss its benefits compared to traditional formulation of buffer solutions.
-
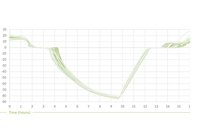
Controlling the freezing behavior of bulk drug substance is the ultimate goal in biopharma manufacturing. This study demonstrates the impact of ice front growth speed on scalability of freezing protein solutions.
-
Supply chain management for CGTs will become increasingly complex to sustain their specific requirements. A much-needed approach to solving problems related to supply chain management is the development of smart packaging and related smart technologies.
-
There is no full Pharma 4.0 manufacturing in place yet, but there are first innovations to build on.
-
Cell and gene therapies yield a small volume of individualized product for each single patient. Loss of the shipment or damage to the transported goods have serious consequences for patients.
-
There is still potential product loss with unprotected single-use bags, and there are still human errors dampening the euphoria of single-use systems. Here's how to protect your product investment.
-
Emerging process solutions provider Single Use Support has announced major investments of 20 Mio EUR in development and research of Biopharma novel process solutions. Its primarily focus is to enhance product safety, expand process capability, and improve speed and effectiveness for production of cell and gene therapies. The Austrian company’s upcoming innovation will be the launch of its own single-use bags – with other process innovations to follow.
-
Single-use technology can simplify the fill/finish process and enable ease of use with custom manifold assemblies, TPE or silicone tubing, and adaptable, widely compatible bags.
-
The Indianapolis-based CDMO has ordered RoSS.FILL CGT to fill lentiviral vectors into small single-use bags. Specially designed for cell and gene therapies, RoSS.FILL CGT is an automated cGMP-compliant filling and filtration platform to dispense small to medium sized volumes of highly valuable drug substances into multiple small single use bags. The technology will be supplied to Genezen’s facility by the end of 2021.
-
Scalability around freezing means the ability to guarantee constant stress on proteins (biopharmaceutical molecules) in all scales, filling volumes and loading scenarios. Read how by standardizing the IFGS – icefront growth speed – for all possible scenarios regardless of the filling volume and type of bags but also regardless of the type of system, i.e. whether LabScale, MidScale or LargeScale, you can fulil this requirement.
-
Less than 0,01% of the plastic waste generated annually worldwide are single-use technologies derived from the biopharma. This portion is subject to strict guidelines, so they’re usually collected, decontaminated and disposed rigorously. Furthermore, manufacturers of single-use technologies are obliged to facilitate the separation of the product into its base materials after use. Therefore, the single-use products are mostly very easy to recycle.
-
The international provider of process solutions for the biopharmaceutical industry, Single Use Support, which was only founded in 2016, is experiencing a massive run on its product innovations.
-
In an extremely complex, competitive and constantly evolving sector such as the biopharmaceutical industry, flexibility is key, not least since it is an immensely varied and compliance-driven field. This is one of the reasons why more and more logistics solutions – covering all steps from filling to freezing, thawing and distribution – are primarily based on single-use technologies. Read more on you can optimize performance and efficiency with single-use technologies.
-
A visual comparison of interfacial stress on frozen single use bags: How bags experience stress in plate-based freezers versus conventional static freezers.
-
SUSupport is a leading provider of safe, reliable and user-friendly solutions when it comes to protected storage and/or shipping of highly sensitive and at the same time highly valuable drug substance contained in single use bags. In addition to their flagship product, the RoSS shell, which protects 2D single-use bags of various sizes, the Austrian solution provider has now developed a stainless-steel 3D bag holder that offers reliable protection for all single use bags of up to 1,000 l.
-
In order to comply with the versatile requirements of today’s market, flexibility and the option to customize are key whilst maintaining availability and short lead times. This is true for many areas, not least when it comes to the ever-growing number of single-use technologies utilized in the biopharmaceutical industry.
-
In response to the changing process intensification, development, and production requirements, more labs are choosing an approach based on recyclable and extremely flexible single-use technologies.
VIDEOS
-
Discover how controlled freezing minimizes cryoconcentration and protein denaturation in bulk drug substances. Learn about efficient, scalable freezing solutions for enhanced product stability.
-
For any single use bag and any freezing infrastructure freeze your drug substance/product without worries! The right material at the right place – what we can learn from oysters. Tough protected & tamper evident! The tried and tested single-use bag gets its 100% secure and compact shell.
DATASHEETS
- Blast Freezing And Thawing For Any Primary Packaging
- RoSS.FILL Bottle: Fill And Filter Your Drug Substance Into Single-Use Bottles
- Accurate Single-Use Bag Filling System For Small Volumes
- IRIS Single-Use Assemblies: Fluid Management Made Simple
- RoSS.PPST: Pre-Use Post Sterilization Integrity Testing
- Fluid Management Made Simple With IRIS Single-Use Assemblies
- RoSS.PADL: Cooling And Homogenizing Cells
- Mid Scale Plate-Based Freeze-Thaw Unit
- Reducing Product Loss To <0.001%
- Smart Tracking For International Cold Chain Transport
- Cryogenic Controlled Freezer
- Scalable Freeze-Thaw Platforms For Single-Use Bags And Bottles
- RoSS.KSET: Protection For Different Single Use-Bags