
RECENT WEBINARS

Translating Stem Cell Programs To GMP
A detailed exploration of challenges in advancing stem cell programs to GMP, highlighting strategies to control variability, strengthen process design, and support reliable clinical‑stage manufacturing.
The Future Of CGT: Integrating Digitalization With Regulatory Readiness
Explore how cell and gene therapy manufacturers are scaling from batch‑of‑one to commercial supply while staying compliant, digital, and efficient, as well as strategies to strengthen quality.

Four Common Pitfalls To Avoid In UF/DF Setup And Scale-Up
Learn how small UF/DF decisions can create major scale-up challenges, as well as practical ways to improve membrane selection, reduce manual operations, and boost PD–MSAT alignment.

Bridging Discovery And CMC With Rapid Pools
Learn how early stable CHO expression data reveals manufacturability issues in complex antibodies that transient screening misses, with case studies showing how this changes lead selection.

Charting The Path To First-In-Human: Strategic Readiness For Early Clinical Success
Gain a comprehensive, end-to-end perspective on the journey to first-in-human and understand how early decisions influence downstream success.

Selecting A Prefillable Syringe System With Confidence
Discover an advancement in the prefillable syringe market, uniquely integrating the syringe barrel, plunger, and needle shield/tip cap into a fully harmonized, verified system from a single supplier.
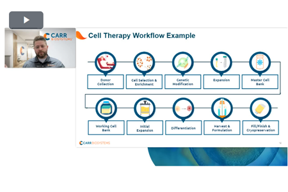
Simplified Harvest And Media Exchange In Cell Therapy
Scale-up often introduces shear stress and process variability. Learn how low-shear, single-use technology stabilizes media exchange and harvest, ensuring consistent cell quality and predictable performance.

Next-Level Cell Lines: An Integrated Approach To Biologic Innovation
See how AI-driven tools and optimized platforms are accelerating biologics development, delivering high-titer clones in weeks, and paving the way for integrated design of cell lines and genetic systems.

Five Practical Considerations To Move From Concept To Clinic
Learn more about five key factors for advancing targeted in vivo LNP programs, from formulation and targeting strategies to bioanalytical readiness and scalability.

Overcoming Regulatory Hurdles In AAV Production
Demonstrating residual reagent clearance is a critical regulatory requirement in AAV manufacturing. Explore how specialized assays and strategic partnerships streamline compliance.

From Lab To Commercialization: Simplifying Bioprocess Scale-Up
Explore how unique cuboid geometries and advanced mixing dynamics create consistent performance from the bench to commercial manufacturing, ensuring flexibility and improved productivity.
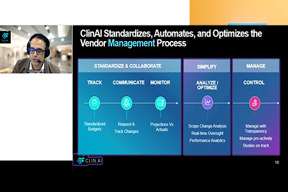
CRO Selection Science: Insights From $2B+ Vendor Choices
Discover how data-driven strategies and structured RFPs can simplify CRO selection, improve transparency, and ensure the right operational fit. Learn practical steps to make smarter vendor decisions.
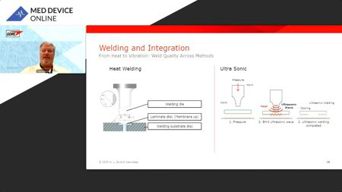
Seal The Deal: Mastering Vent Integration In Medical Devices
Learn how precise vent placement and microfiltration design optimize performance in life science devices. See practical troubleshooting strategies and a real-world case study.

Lessons From FDA 483s And Warning Letters: Cleanroom Compliance
Learn critical lessons from FDA 483 observations and Warning Letters to proactively address common GMP cleanroom compliance failures and build an inspection-ready facility.
Robust And Cost-Effective mRNA Capping
Master the essential strategies for cost-effective and robust mRNA capping at industrial scale. Learn how to successfully evaluate new materials and optimize your synthesis process for maximum efficiency and yield.

Eliminate Risk From Your Viral Vector Tech Transfers
Explore strategies to simplify viral vector tech transfers, reduce risk, and maintain quality under tight timelines, as well as a case study that demonstrates how to streamline this critical process.

Navigating Innovation And Challenges In CGT Bioprocessing
This expert panel breaks down the practical realities of today’s supply chain while forecasting how emerging technologies will redefine quality standards.

Navigating Regulatory Expectations For Injectable Packaging
Explore insights on extractables, focusing on how they relate to the revised EU GMP Annex 1, new and evolved expectations under USP <382>, and the use of VHP decontamination in aseptic environments.

Cleanroom Changes In 2026 For Better Contamination Control
Proactively improve your cleanroom strategy for 2026. Gain actionable insights on leveraging Environmental Monitoring data, optimizing personnel, and managing residue to enhance contamination control.

Critical Path For Gene Therapy: AAV Analytical Lifecycle Considerations
Explore considerations for phase-appropriate AAV characterization and release activities from pre-clinical to late-phase products. Review validation challenges and paths for maturation of analytics.

Raising The Bar In Gene Therapy
Learn how a science-driven and digitally structured approach reduced onboarding timelines for gene therapy from 12 months to just 3, setting a new benchmark in technology transfers.
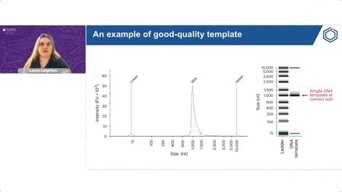
The mRNA Journey: From Design To LNP-Ready Molecules For Research
Learn a validated, end-to-end protocol for producing high-quality in vitro transcription (IVT) mRNA for preclinical research. Find out how to optimize sequence design, small-scale synthesis, and more.
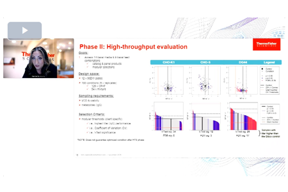
Smart Media Screening And Design: Saving Time And Boosting Titers
Optimizing your cell culture media selection is crucial for clinical advancement. Learn how high throughput screening techniques accelerate media and feed optimization to significantly boost your biologics' titers.

Addressing The Challenges Of LNP Upscaling
Overcome LNP upscaling challenges by leveraging fluid mechanical principles. Learn how Impingement Jets Mixing achieves reproducible turbulence and consistent LNP quality across all production scales.

Streamlining pDNA: Capacity, Complexity, And Cutting-Edge Solutions
Learn how flexible facility design and process innovation are accelerating pDNA manufacturing, as well as key strategies to avoid scale-up pitfalls and meet growing therapeutic demand.

Are You Keeping Pace With Oligo Synthesis Optimization?
The rapidly expanding oligo market demands optimized RNA synthesis. Examine key innovations and workflow insights to maximize resources and control long-term manufacturing costs.

Accelerate Your pDNA And mRNA Process Development
Explore a structured approach to optimizing and scaling plasmid DNA production using E. coli, with insights into key growth parameters, benchtop feasibility, and pilot-scale performance.

Practical Solutions For Protein Analytics And Residual DNA Testing
Discover practical solutions for common quality control bottlenecks in biotherapy manufacturing. Learn how to improve protein analytics and DNA testing robustness and accelerate time to market.

Choosing The Right Sporicide: Critical Factors For Robust Cleanroom Contamination Control
Choosing the best sporicide for your cleanroom involves evaluating factors like surface format, contact time, and safety. Learn the criteria to select an effective and robust agent for contamination control.
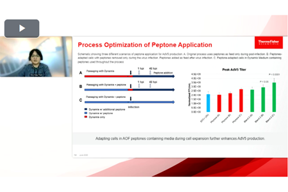
Boosting Vaccine Yields Through Smarter Cell Culture Media Optimization
Unlock efficient vaccine production by understanding how tailored peptone supplementation optimizes cell culture media, improving cell growth and overall yield across multiple critical cell lines.
