
CELL AND GENE THERAPY ANALYTICS

Interview With Pharma Analytics Field Application Specialist - Sandi True
Hear Sandi True, a Field Applications Specialist discuss helping customers navigate challenges often encountered when evaluating and implementing Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.

Multi-Omics And Bioinformatics In Cell Culture Media Design
Learn about the impact of a multi-omics analysis, utilizing proteomics and metabolomics, in the development and optimization of media with the goal of improving titer, and making development and manufacturing more efficient.

Simple In-House Mycoplasma Testing Method For Regulatory Expectations And Rapid, Confident, And Actionable Results
In this webinar, we present an overview of worldwide regulatory guidance for mycoplasma testing, as well as a mycoplasma testing method that has been accepted by regulatory authorities worldwide across various therapeutic modalities.

Rapid Micro Methods In QC Micro Testing: A NIBRT Perspective
Learn about some of the advanced Rapid Micro Method systems that are being adopted by biopharma companies worldwide, and how they are changing the face of QC Microbiological testing.

Innovative Contamination Control: Enabling Integrity And Efficiency
An integrated approach can enhance contamination control, boost process efficiency, and ensure the production of high-quality cell therapy products.
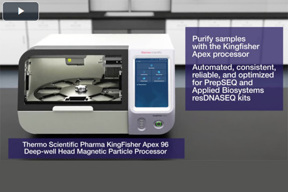
ResDNASEQ Workflow Solution
Explore the Applied Biosystems resDNASEQ workflow solutions. Starting with the sample preparation kit, to purification, quantitating residual host-cell DNA, and analyzing resDNASEQ assays.

Scaling Viral Vectors
Industry experts cover the high operational costs associated with viral vectors, along with the challenge presented by their shot shelf life and scalability.

Interview With Nico Chow And Sandi True, Field Applications Specialists
Hear Nico Chow and Sandi True, Field Applications Specialists, discuss working with customers to help them evaluate and implement Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.

Cell And Gene Therapy Innovations
This interview with a pharmaceutical analytics expert discusses key insights into advances in cell and gene therapy and the strategies used to support customers in improving their therapeutic development.

Successful Microbial Testing And Identification
In this presentation, one of our industry-leading customers discusses how they've successfully implemented the Applied Biosystems MicroSEQ Microbial Identification System in their laboratory testing.

Analytical Solutions To Help Accelerate Bioprocessing Success
Unlock the power of data-driven bioprocessing. Discover how advanced analytics can optimize your cell culture process, improve performance, and accelerate success.

Manufacturing Hurdles With Viral Vectors
With a wide range of viral vector-based drugs already approved, viral vectors are expected to remain the primary delivery mechanism for the foreseeable future. However, as the demand for viral vectors increases, addressing the challenges related to their manufacture and s...

Innovative Strategies For Residual DNA And Viral Titre Quantitation
Discover how digital PCR improves the accuracy of residual host cell DNA and viral titre quantification in biotherapeutic manufacturing to enhance product quality, safety, and regulatory compliance.

A Straightforward Path Toward Regulatory Compliance, Data Integrity, And Computer Systems Validation
Learn practical strategies for validating microbial identification systems in cGMP. Understand regulatory guidance and master a complete Computer Systems Validation plan for full compliance.
Maximize Quality Assurance Through Rapid Sterility Testing
This webinar explores mitigating the risk of product loss, and production delays, as well as the critical role rapid sterility testing plays in ensuring the quality and safety of cell therapy products.

Microbial Identification Via DNA-Seq
Learn how DNA sequencing-based microbial identification supports regulatory compliance and enhances contamination control in critical environments.

Quality Control Assays
Two company leaders detail not only the vectors their respective companies use and why but also the quality control assays performed on viral vectors.

Validation Of A qPCR Assay For Host Cell DNA Quantitation
Here, we share the development and validation of a new, highly sensitive and accurate integrated solution for detection and quantitation of host cell DNA to help meet regulatory requirements.

A Straightforward Path Toward Regulatory Compliance & Data Integrity With Your Microbial Testing Systems
In this webinar, we will share a variety of strategies for implementing and validating microbial identification systems in the cGMP environment, possible difficulties along the way, and a comprehensive solution that addresses these challenges.

Explore The Analytics Knowledge Hub
Discover smarter solutions with the new Analytics Knowledge Hub that provides articles, webinars, e-books, and infographics designed to enhance and streamline your bioprocess workflow.

Inside Bioprocessing: Multi-Omics
Dive into multi-omics analysis with Paul Gulde, Chengjian Tu, and Femi Egbebi. In biopharmaceutical production, meeting the unique nutrient requirements of the cells is central to achieving optimal results.

Multi-Omics In Cell Culture Media Design
Discussing the evolution of medium design for cell culture bioprocessing, and the role that multi-omics and bioinformatics can play in improved medium development.

Rapid Process Development And Technical Support For AAV Scaleup
Accelerate your AAV production journey. Learn how rapid process development and expert support can streamline your path from vial to purified bulk, ensuring scalable and efficient manufacturing.

Regulatory Compliance And Viral Vectors
Hear from experts who detail the specific regulatory challenges for advanced therapies, as well as how the regulatory requirements differ in early research and development in academia and in industrial vector production.

Market Demand For AAV Vectors
Here, Dave Maheu, VP, Head of Process & Analytical Development at Candel Therapeutics and Curran Simpson, Chief Operating Officer at REGENXBIO detail the key factors driving the adeno associated virus manufacturing market.

An All-In-One Solution For Residual DNA Quantitation
Explore an all-in-one solution for residual DNA quantitation with a 3D lab tour with virtual demos, videos, and interactive instrument guides to experience the full workflow.

Analytical Approaches For Quantifying Residual Host Cell DNA
In this webinar, we’ll discuss the challenges, risks and considerations involved in developing and using in-house residual testing solutions, and the benefits of using commercial kits for residual DNA testing.
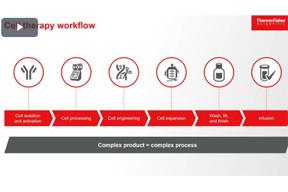
Leveraging Rapid Sterility Testing To Advance Cell Therapy Production
Explore the crucial role of rapid sterility testing in cell therapy manufacturing. This presentation delves into the benefits of swift, accurate detection for product quality and patient safety.
Implementing Rapid Microbial Identification In Biotherapy Manufacutring
Learn about rapid microbial identification strategies that enhance environmental monitoring and compliance with regulatory requirements for your manufacturing processes.

Overcoming Capacity Constraints
How can CGT biotechs overcome issues such as building an in house facility and ultimately get their therapies to patients.
