Inside Cell & Gene Live "2024 Regulatory Outlook With FDA's Dr. Peter Marks And Dr. Nicole Verdun"

By Erin Harris, Editor-In-Chief, Cell & Gene
Follow Me On Twitter @ErinHarris_1

In case you missed it, our recent Cell & Gene Live, 2024 Regulatory Outlook with FDA’s Dr. Peter Marks and Dr. Nicole Verdun, is available on demand. During the 45-minute presentation, Dr. Marks and Dr. Verdun shared valuable information about various regulatory topics that can and will impact the cell and gene therapy sector this year and in the near future.
We began the presentation by discussing the new Office of Therapeutic Products, the new super office, which is the result of a reorganization of CBER’s Office of Tissues and Advanced Therapies. I asked Dr. Verdun, Super Office Director, about the plans the Office of Therapeutic Products has in store for 2024. As part of her response, she shared, “We really are invested in trying to get more of the rare diseases where there is a significant unmet need, and to get therapies to the market for rare disease. And so that really is one of the pillars and one of the things that you will hear Dr. Marks and I talk a lot about in various capacities is really getting that going even more in 2024.” She also shared they will be “increasing operational efficiencies, hiring, and getting people on board and up to speed; getting to a place where we’re also connecting an increased engagement with external stakeholders who really are quite important in this partnership.”
Next, we spent some time discussing how the learnings from approved gene therapies might pave the way for future approvals. As part of a more detailed response to their learnings, Dr. Marks stated, “One, the importance of manufacturing. Two, the importance of leveraging appropriate accelerated approval endpoints when we can. And three, the importance of communications both with sponsors and with our global regulatory counterparts.”
CMC was a key topic, and one we devoted quite some time to during the presentation. I asked our panelists about the developments they expect to see this year in CMC for advanced therapies. Dr. Marks offered a detailed explanation. He began by stating, “Right now, the process is not highly efficient, it's not standardized, and that's leading to a lot of wastage in terms of time and money as products come forward from initial study in very small populations to potential commercial product candidates. And so hopefully during this year we'll see some transformation in that regard.”
When it comes to challenges demonstrating CMC comparability during late-stage product development, Dr. Marks added, “We mentioned this earlier, but the development of a potency assay. I think that I want to just touch on that a bit. I think the earlier in product development that you can develop a potency assay, I do think that the better. I think that in terms of comparability, what we sometimes see is the product that's used in development there then is a shift in terms of some pieces of the manufacturing that vary from the product use and development to the commercial product that is proposed. And so, I do think that some of the challenges that we see are not considering this early enough in the product development such that we're now in a place where we don't have assays in place or other pieces to truly get to comparability. So, I would just encourage sponsors to think through some of these potential challenges early on and plan for that as much as you can so that we aren't in a space where we're trying to just make sure that the clinical data that was collected on one form of the product is applicable to what we're wanting to do in the commercial setting. But I agree it's a challenge. I think it's something again where communication is key because the more we can talk about the individual circumstances, we can get to some resolution here.”
In addition, we covered advice for small and emerging biotechs, AI and machine learning’s impact on the regulatory landscape, and much more. We also took questions from our audience – all of which can be viewed during the full-length presentation. If you aren’t already a subscriber to Cell & Gene’s e-newsletter, be sure to register. In the coming days and weeks, we will include brief video clips by topic from this important Cell & Gene Live. The video clips are a great way to view the entire presentation one topic at a time and according to your busy schedule.
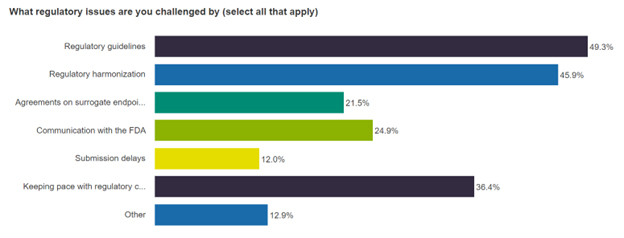
Finally, during each Cell & Gene Live, we poll our audience to better understand their take on the topic at hand. For your reference, here are the results that were generated in real time about the audience’s most pressing regulatory issues or challenges. Are you and your team facing the same challenges? Email me to let me know.