Key Considerations For Developing A Cell Therapy Manufacturing Roadmap
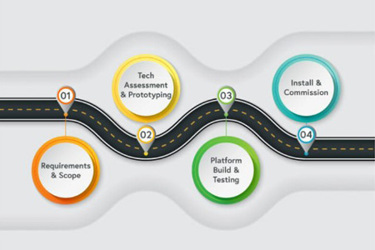
Cell therapy manufacturing is a rapidly growing field that presents new challenges. Creating a manufacturing roadmap can help your organization navigate through these challenges and act as a guide to automation implementation. This roadmap should be developed during the preclinical phase and is a dynamic, flexible document requiring ongoing refinement. To create an effective roadmap, manufacturers must outline the entire process and understand both immediate and future manufacturing needs. Factors such as project scale, existing automation options, process variabilities, manufacturing costs, and process validation also need to be considered. By staying aware of industry advancements and utilizing the latest technologies, manufacturers can evolve with changing needs and contribute to the success of cell therapies. The roadmap can enhance your understanding of short and long-term manufacturing requirements and help you identify opportunities for process optimization.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
