Designing A Patient-Centric Digital Ecosystem: Strategies For Cell And Gene Therapy CxOs
By Josh Fyffe, Senior Consultant and Rajesh Singh, Principal, Deloitte Consulting LLP Contributors: Hussain Mooraj, Principal, and Jeffrey Lacey, Senior Manager, Deloitte Consulting LLP

Cell and gene therapy (CGT) production is costly, complex, and high-risk; in part, because most supply chain and manufacturing processes involve multiple stakeholders and facilities. In the first article of this series we discussed how CGT adoption and success hinges on early planning to scale manufacturing and supply. This article describes strategies to enable a patient-centric digital ecosystem and capabilities to support CGTs before, during, and after the order life cycle.
Over the last 5+ years, CGT production has spawned new clinical and commercial norms, connections, and partnerships across the life sciences and health care landscape. To deliver an end-to-end, autologous or allogeneic therapy, a host of disconnected parties need to navigate challenges and constraints throughout the order life cycle, such as ensuring the chain of custody/chain of identity (COC/COI), aligning key handoffs, and establishing and centralizing order transparency. Increasingly, CGT suppliers have addressed these pain points and improved process efficiency by funding, designing, and deploying order life cycle digital ecosystems.
Patient enrollment, scheduling, and materials tracking are among the life cycle capabilities that many CGT suppliers already have digitized and standardized. However, therapy-specific differences, market access considerations, and patient journey improvements continue to shift CGT clinical and commercial business processes, driving suppliers to invest in innovating and evolving their CGT digital capabilities (figure 1).
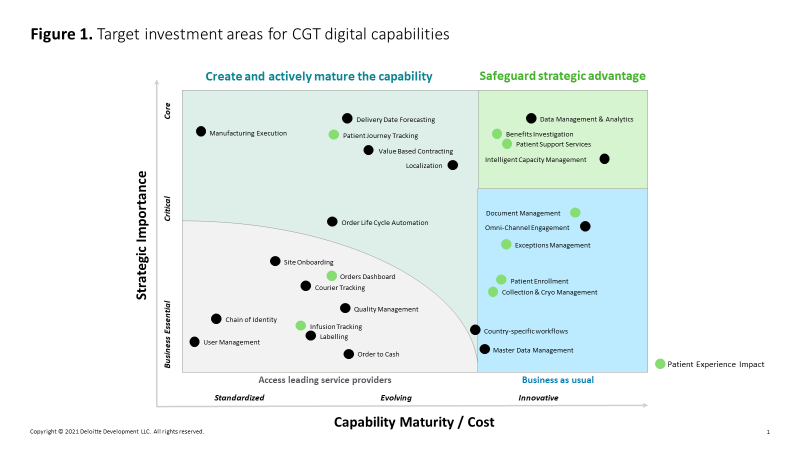
As CxOs and other senior executives develop strategies to cost-effectively mature their organization’s digital ecosystem, it is important to consider both the specific technologies and the broader information technology (IT) infrastructure needed to support CGTs. We suggest CxOs begin by addressing the following questions:
- What is our IT strategy for a CGT launch?
- Are we engaging cross-functionally internally and collaborating externally to inform solution development?
- How do we manage future releases for sustained excellence?
- How can we evolve our digital ecosystem to better serve patients?
What is Our IT strategy for a CGT Launch?
Launching a CGT offering is inherently complex, especially when conducted on a global scale. The following strategic planning steps should include CxO input to enable future digital readiness:
- Align on target objectives (i.e. raising and creating market awareness for the product offering).
- Identify a launch timeline (This may include a pathway to qualifying and certifying treatment sites, enabling a digital asset to meet market requirements, and aligning business regulatory requirements.)
- Identify team roles and responsibilities across the launch.
- Identify local subject matter experts (SMEs) who can validate design and test the solution throughout the timeline.
- Define workshop cadence with SMEs to align with timeline and scope inputs.
In addition, the CIO/CTO organization should contribute to the master playbook that delineates shared and separate steps to launch a CGT product. The following are among the IT-related actions collated across several market leaders:
- Create a standardized baseline and reference for all markets and identify additional market-specific variations.
- Generate a capability blueprint and conceptual architecture, and determine logical data flow.
- Select from build, acquire, and partner options to obtain the required digital capabilities.
- Collate minimum viable regional requirements across business processes to inform IT solution impact.
- Evaluate existing digital landscape and enablement against market needs to identify if technology stack supports all regional requirements (e.g., data localization and residency requirements).
- Produce a timeline to design, build, test, and deploy that fits to overall business launch objectives.
- Identify business representatives familiar with local market requirements and processes to guide and partner with IT throughout the deployment phase.
- Plan and design solutions that can manage processes that deviate from the happy path (e.g., out-of-specification management).
- Budget and plan for resources to deliver required changes to access new markets.
- Coordinate with hospitals/treatment centers to ensure that they have adequate infrastructure to enroll patients, perform apheresis, and infusion.
Are We Engaging Cross-Functionally Internally and Collaborating Externally to Inform Solution Development?
While a playbook maps the required IT actions and digital capabilities needed to launch a CGT or expand its market presence, it is only as effective as the individuals and groups working together to move each action forward.
Developing and evolving a CGT digital ecosystem leans heavily on two distinct IT talent pools: Build teams that scope, build, design, test, and deploy the CGT digital platform; and Operate teams that run and use the platform and its applications. Engaging early and often with each other and with the organization’s commercialization, supply chain, and finance functions (among others) can produce more informed digital solutions.
CGT Build teams typically address IT delivery workstreams, change management, project management, market access, and program management to create a well-coordinated IT delivery and business go-live. In addition to IT staff, a Build team should include business product owners to enable smooth process handovers throughout the CGT order life cycle.
CGT Operate teams generally focus on digital process resiliency, operational, and customer experience improvements. Seeking input from commercial and supply chain representatives at the beginning of ecosystem development and prior to subsequent iterations can help identify latent needs that may require unplanned-for system capabilities.
The nature of a CGT product also requires collaborating with external partners including hospitals, contract manufacturing organizations, couriers, and others to optimize the product flow, including multiple hand-off points, to provide a differentiated patient experience.
Communicating, collaborating, and iterating within the Build and Operate teams, with other organizational functions, and external stakeholders can produce a robust digital roadmap and healthy project backlog that spans the CGT order life cycle.
How Do We Manage Future Releases for Sustained Excellence?
Project scope for the initial release of an order life cycle digital ecosystem may be limited to technologies that support foundational capabilities that will be used across markets and therapies, or those that deliver significant return on investment. Examples include customer relationship management (CRM), cloud-based software, middleware, and enterprise resourcing planning (ERP) systems. Several questions can help frame the required functionality:
- Without this feature, can we go-live within the desired market?
- Is this feature required to facilitate an essential business process in the order life cycle?
- Does this feature offer a leading customer or operational benefit?
- Is there an operational risk via data integrity, product quality, or patient safety if this feature is not enabled?
These and similar questions can also guide a CxOs plans for subsequent market rollouts and enhancement releases that build upon the foundational capabilities selected for the first go-live (figure 2).
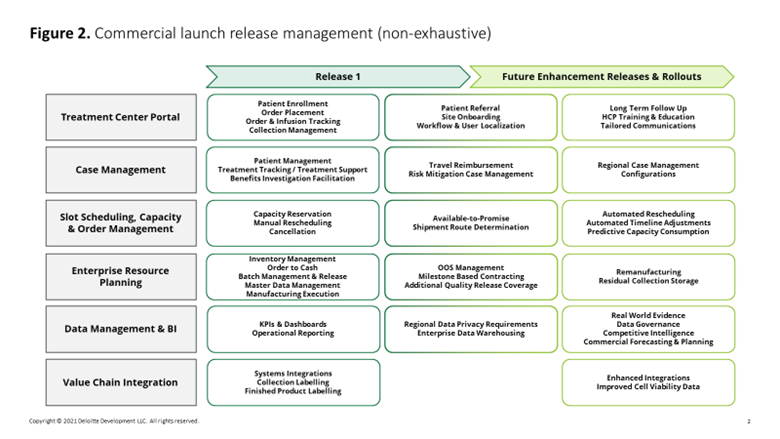
Two lean budgeting and cost management approaches can help maximize the value of future releases:
- Economies of scale: Conducting proper country due diligence can drive rollout savings. Some markets have matching regulatory requirements for ordering, tracking, and infusing blood products. If one of these markets is enabled digitally, the rest can be enabled in parallel if the business is aligned to the position. The incremental cost to deploy one or two of these countries versus 10 (assuming like-requirements) is insignificant.
- Efficient dev ops model: Within weeks of a business go-live of a digital solution, users will begin generating enhancement requests. Sponsoring a new release for these ongoing enhancements may have a larger cost than investing in an annual dev ops team to facilitate small enhancement delivery on monthly or bi-monthly basis. This will depend on many factors, however assessing a dev ops model and associated rates for enhancements is recommended.
How Can We Evolve Our CGT Digital Ecosystem to Better Serve Patients?
As pharmaceutical company portfolios shift to include more CGTs and historical organizational structures converge with fit-for-purpose CGT operating models, how can CxOs and their teams evolve today’s CGT digital ecosystems to enable future therapies and better serve patients? The process should begin with the CxO establishing clear ownership of the supplier’s digital assets and their future development. This will centralize authority with the CxO to set scope and address issues such as:
- How is funding confirmed and sourced for the organization’s cross-functional products and digital assets?
- How can we create an operating structure that successfully blends the required talent to deliver these applications and products?
- How do we break down silos to achieve collective alignment on process and solution at the beginning of each evolution?
- What defines the success of this CGT solution? What is the definition of good?
The years that CGTs have been in the market can be counted on two hands. This is a new, rapidly changing space that calls for innovative operating models, manufacturing processes, and seamless internal/external stakeholder collaboration to disrupt the traditional supply chain. A well-designed digital ecosystem can be the key enabler to a differentiated patient and caregiver experience.
About The Authors
 Hussain Mooraj is a principal and leads Deloitte Consulting LLP’s NextGen Therapy practice and is the New England regional lead for life sciences. He brings more than 25 years of experience in manufacturing, supply chain, enterprise technology, sales and marketing, and strategy consulting to his role. Mooraj works closely with senior executives from global life sciences firms, helping them transform their end-to-end businesses and build startup organizations to be able to launch lifesaving new therapies, especially on the CAR-T and gene therapy side.
Hussain Mooraj is a principal and leads Deloitte Consulting LLP’s NextGen Therapy practice and is the New England regional lead for life sciences. He brings more than 25 years of experience in manufacturing, supply chain, enterprise technology, sales and marketing, and strategy consulting to his role. Mooraj works closely with senior executives from global life sciences firms, helping them transform their end-to-end businesses and build startup organizations to be able to launch lifesaving new therapies, especially on the CAR-T and gene therapy side.
 Josh Fyffe is a senior consultant in Deloitte Consulting LLP’s Customer and Marketing practice, focusing on life sciences and cell and gene therapies. In the last four years, Fyffe has worked closely with biopharma companies to define system architectures, capabilities and roadmaps for CGT products through scoping workshops, SME engagements, industry certification requirements, and global regulatory considerations. Josh maintains a detailed understanding of these requirements, technical applications and processes to inform client-specific architectural and design considerations.
Josh Fyffe is a senior consultant in Deloitte Consulting LLP’s Customer and Marketing practice, focusing on life sciences and cell and gene therapies. In the last four years, Fyffe has worked closely with biopharma companies to define system architectures, capabilities and roadmaps for CGT products through scoping workshops, SME engagements, industry certification requirements, and global regulatory considerations. Josh maintains a detailed understanding of these requirements, technical applications and processes to inform client-specific architectural and design considerations.
 Rajesh Singh is a Managing Director in Deloitte’s Life Science practice and leads Technology for NextGen Therapy. He has over 18 years of industry and consulting experience in the bio-pharmaceutical sector in Supply Chain, Manufacturing, Enterprise Technology and Architectures, Governance and Portfolio Management. Specifically for Cell & Gene therapy, he is currently advising several early and mid-stage companies on critical system strategies and leading large-scale global implementations for late-stage ones.
Rajesh Singh is a Managing Director in Deloitte’s Life Science practice and leads Technology for NextGen Therapy. He has over 18 years of industry and consulting experience in the bio-pharmaceutical sector in Supply Chain, Manufacturing, Enterprise Technology and Architectures, Governance and Portfolio Management. Specifically for Cell & Gene therapy, he is currently advising several early and mid-stage companies on critical system strategies and leading large-scale global implementations for late-stage ones.
About Deloitte
Deloitte refers to one or more of Deloitte Touche Tohmatsu Limited, a UK private company limited by guarantee (“DTTL”), its network of member firms, and their related entities. DTTL and each of its member firms are legally separate and independent entities. DTTL (also referred to as “Deloitte Global”) does not provide services to clients. In the United States, Deloitte refers to one or more of the US member firms of DTTL, their related entities that operate using the “Deloitte” name in the United States and their respective affiliates. Certain services may not be available to attest clients under the rules and regulations of public accounting. Please see www.deloitte.com/about to learn more about our global network of member firms.
This publication contains general information only and Deloitte is not, by means of this publication, rendering accounting, business, financial, investment, legal, tax, or other professional advice or services. This publication is not a substitute for such professional advice or services, nor should it be used as a basis for any decision or action that may affect your business. Before making any decision or taking any action that may affect your business, you should consult a qualified professional advisor. Deloitte shall not be responsible for any loss sustained by any person who relies on this publication.
Copyright © 2021 Deloitte Development LLC. All rights reserved.
