Sera Products For Cell And Gene Therapy
Corning’s expert manufacturing and reliable supply of serum products perfectly complement our classical and custom cell culture media portfolio.
Our serum products are collected, processed, and tested in accordance with cGMP standards and EP and USP methods. They are certified for traceability and suitability by the ISIA and EDQM.
Products Include
- Custom Media and Serum
- Bovine Calf Serum
- Fetal Bovine Serum
- Horse Serum
- Rabbit Serum
- Sheep Serum
- Custom Media and Serum
Corning's Vertically Integrated Serum Supply Chain
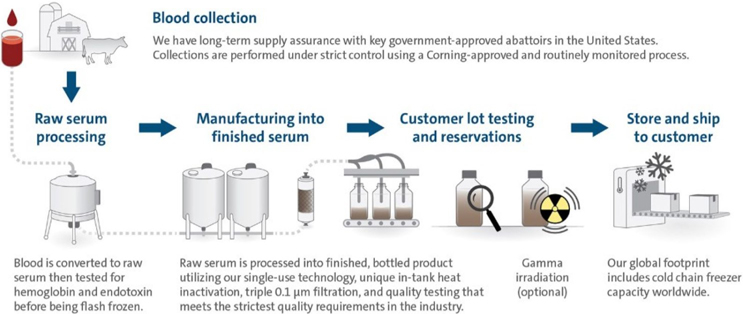
Origin
We offer several premium FBS (Fetal Bovine Serum) products collected in approved abattoirs from herds in the United States, Australia, and New Zealand. Also, our regular FBS (Fetal Bovine Serum) is collected in approved facilities from herds located in countries approved by the USDA for importation into the United States. In certain regions we also provide South America-sourced FBS (Fetal Bovine Serum) manufactured at facilities approved by the appropriate local authorities.
Sterile Filtered
The raw serum is filtered through a series of three 0.1 micron sterilizing filters. Corning requires each serum lot to test negative for mycoplasma by two methods to qualify as product for our customers.
Low Endotoxin Levels (LAL) Limulus Amebocyte Lysate
Each lot is tested for bacterial endotoxin. Our premium grade FBS (Fetal Bovine Serum) must meet a specification of ≤5 EU/mL and our regular grade FBS (Fetal Bovine Serum) must meet a specification of ≤20EU/mL.
Low Hemoglobin Levels
A quantitative and colorimetric assay is performed to determine the residual hemoglobin concentration in each product lot. All lots of premium Corning FBS (Fetal Bovine Serum) are verified to contain ≤25 mg/dL of hemoglobin while regular grade contains ≤30 mg/dL of hemoglobin.
Certificate of Analysis and Biochemical Profile
Corning sera products are analyzed for quality and performance. Testing includes pH, osmolality, growth promotion, sterility (current USP method), mycoplasma, total protein, endotoxin and hemoglobin levels, virus, proteins, hormones, and trace medals.
EDQM Certificates of Suitability
Fetal Bovine Serum, United States Origin
This product has been granted a Certificate of Suitability, R0-CEP 2018-271, by the European Directorate for the Quality of Medicines (EDQM) certifying that the product meets the criteria described in the current version of the monograph no. 1483 of the European Pharmacopoeia “Product with risk of transmitting agent of animal spongiform encephalopathies.”
Bovine Calf Serum, United States Origin
This product has been granted a Certificate of Suitability, R0-CEP 2018-305, by the European Directorate for the Quality of Medicines (EDQM) certifying that the product meets the criteria described in the current version of the monograph no. 1483 of the European Pharmacopoeia “Product with risk of transmitting agent of animal spongiform encephalopathies.”
Fetal Bovine Serum, Australian Origin
This product has been granted a Certificate of Suitability, R0-CEP 2020-127, by the European Directorate for the Quality of Medicines (EDQM) certifying that the product meets the criteria described in the current version of the monograph no. 1483 of the European Pharmacopoeia “Product with risk of transmitting agent of animal spongiform encephalopathies.”
