Biocontainment: An Introduction To Control Levels & Practical Design Concepts
By Herman F. Bozenhardt and Erich H. Bozenhardt
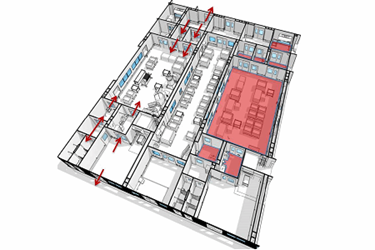
In part 1 of our article on biocontainment, we discussed the history of biocontainment as an outgrowth of medical research, especially the effort to develop vaccines for various worldwide diseases. We provided some definition of the biosafety levels (BSLs) established by the Centers for Disease Control and Prevention (CDC) based on risk considerations. We also discussed the regulatory regime for biological agents and, specifically, for designing a facility for an FDA or EU regulated product within the confines of the safety practices dictated by the CDC and design requirements of the NIH.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
