
MANUFACTURING

Single-Use Filter Transfer Sets Ready For Final Filling
Our industry-first, preconfigured filter transfer set for final filling arrives up to 80% faster, is PUPSIT-optimized, sterile and ready to use.
Assuring Viral Safety: Better, Faster, Virus Production
Viral safety is tricky when your product is a virus. Learn about the strategies that can help prevent, detect and remove the “bad” viruses from your virus production processes.

Small-Scale Oligonucleotide Synthesizer That Supports Scale Up
Learn about a small oligonucleotide synthesizer with big impact that features a user-friendly interface, compact footprint, and more. This small scale system is user friendly and supports scale up.

Introducing Our Single Use Mixing System
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our single-use mixing system made to be deployed rapidly to help manufacturers meet development, supply, and cost objectives.
Microcarrier Separation Systems
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our highly functional and adaptable microcarrier cell separation bags designed for single-use processes.

Reimagine Sustainability In Bioprocessing
Achieving sustainability in bioprocessing labs presents distinct challenges. Learn how to reduce your environmental footprint while maintaining efficiency and avoiding extensive re-validation efforts.

Freezing Studies For Single-Use Solutions: Long-Term (Part 2)
Watch part 2 of a four-part series, "Four Common Types Of Cryogenic Studies For Advanced Therapies," to learn about long-term freezing studies.

MilliporeSigma Large Scale Capabilities Tour
A detailed look at MilliporeSigma’s new BioReliance® viral vector manufacturing facility and how we have employed the latest innovations and quality systems to deliver best-in-class gene therapy services.

Biopharma Resilience: Bionova Scientific Talks Capacity, Speed, And Agility
Bionova Scientific shares how a CDMO can offer biopharma companies the flexible capacity they need to meet the growing needs for medicines today and in the future.

Key Functions Of The ÄKTA Oligosynt Synthesizer
Discover a compact, fully automated oligonucleotide synthesizer for robust, scalable process development. Learn how it can help establish a robust and reproducible oligo synthesis process.
Freezing Studies For Single-Use Solutions
Learn about the four common types of cryogenic studies for advanced therapies: controlled-rate, long-term, therapy containment/protection, and transportation.
Solutions To Streamline Your Engineered Immune Cell Analytics
An essential component of development and manufacturing workflows is the accurate and standardized analysis of engineered immune cells. Explore solutions designed to streamline these processes and ease every step.
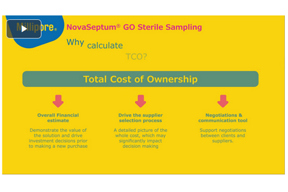
NovaSeptum® GO Sterile Sampling: Total Cost Of Ownership
Risk of contamination associated with traditional sampling methods using SIP valve/welding can have a financial impact on your process sampling plan. Find out how much NovaSeptum® GO sterile sampling system can save you by reducing operator related costs while drastic...

Freezing Studies For Single-Use Solutions: Therapy Containment/Protection (Part 3)
Watch part 3 of a four-part series, "Four Common Types Of Cryogenic Studies For Advanced Therapies," to learn about therapy containment/protection studies.

Sampling In The PBS Mini
Learn how to accurately sample from the PBS Mini bioreactor. Discover critical steps for representative samples, including optimal mixing and collection techniques.

Ori Spotlight: How Early Automation Strategies Reduce Costs And Improve Patient Access
A pioneer in regenerative medicine reveals how to bridge the gap from research to reality. Learn how early automation and strategic partnerships can slash costs and expand patient access to therapies.
Cell Insights By Umetrics® Studio
Cell Insights by Umetrics® Studio simplifies advanced data analytics for bioprocessing, offering hybrid modeling, virtual bioreactor simulations, and cell insights to streamline development, and support data-driven decisions.
Biopharma Resilience: Building Biomanufacturing Capacity
Building back biomanufacturing resilience must remain a top priority to secure global life science supply chains. Learn how Arranta Bio is helping prepare the biopharma industry for future demand.

The Future Of mRNA Manufacturing
Watch to explore a box solution that provides flexible and scalable options for your mRNA workflows and is designed to support the evolving landscape of mRNA production.

Single-Use Manifolds For Operational Efficiency
Learn how single-use manifolds can optimize your biomanufacturing processes, reducing time, space, and resources while increasing flexibility, product quality, and scalability.
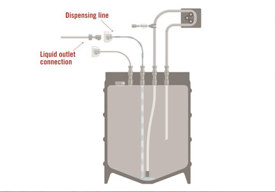
Understanding Our Single-Use Mixing System
Watch how our single-use mixing system creates a completely sterile mixing and recirculation process, ensuring your buffer and media are mixed correctly and efficiently.

How MilliporeSigma Promotes Quality And Risk Reduction
Learn how MilliporeSigma’s new facility was built with quality and risk reduction at the top of mind and its experts have the experience to support large-scale programs through commercialization.

Freezing Studies For Single-Use Solutions: Controlled-Rate (Part 1)
Watch part 1 of a four-part series, "Four Common Types Of Cryogenic Studies For Advanced Therapies," to learn about controlled-rate freezing studies.

Combining Expertise With Best-In-Class Technology
Members of the MilliporeSigma leadership team reflect on the longstanding experience of our BioReliance® viral vector manufacturing facilities. Our legacy experience, alongside our brand new facility and latest industry innovations, ensure we are a preferred partner t...
Antisense Oligonucleotide Extraction Optimization Reveals Differences
Our findings provide critical insights into refining ASO extraction protocols, which can enhance the accuracy of downstream analyses and support further research into ASO-based therapeutics.

How To Maintain A Nuclease Free Environment During mRNA Production
mRNA are singled-stranded, and their chemistry makes them more susceptible to enzyme degradation than double-stranded DNA. Learn how to maintain a nuclease free environment during mRNA production and purification.

Closing Ancillary Processes: The New Bottleneck In Cell and Gene Therapy
One of the continued challenges for scalable commercial manufacturing of cell therapies is closing and automating the entire manufacturing process. Many core manufacturing processes may be closed, but peripheral systems e.g. media formulation, viral vector packaging remai...

Biopharma Resilience: CDMOs Secure Supply Chain Management
Learn how SanKav Pharmaceuticals provides available medicine to market through available components, resources and raw materials that support on-time production lines and biomanufacturing processes.

Bio4C ProcessPad™ Product Overview
Introducing Bio4C ProcessPad™ Software, part of the BioContinuum™ Platform.

Inside An Onsite Test Lab
Learn about the extensive testing and validation to ensure product performance based on risk assessments performed under CPC’s ISO 13485 quality system, streamlining approval processes and allowing manufacturers to reach market faster and with less work.
