Why We Need A New Model To Appropriately Value The Promise Of Regenerative Medicines & Advanced Therapies
By Janet Lambert, CEO, Alliance for Regenerative Medicine
We are in the midst of a revolution in healthcare. New and innovative regenerative medicines and advanced therapies are already helping thousands of patients worldwide, and scores of additional products are in the development pipeline. These therapies are significantly different from traditional medicines, and they are challenging our existing paradigms of healthcare value.
are already helping thousands of patients worldwide, and scores of additional products are in the development pipeline. These therapies are significantly different from traditional medicines, and they are challenging our existing paradigms of healthcare value.
Regenerative medicines and advanced therapies, which include gene and cell therapies along with tissue-based products, can provide patients with profound, durable, and potentially curative treatments, often with just a single administration. In the past five years, we have seen the potential for a therapy to address the underlying cause of a disease and – in many cases – to treat or cure patients who have historically had few or no effective treatments available to them beyond palliative care.
With durable or curative therapies near at hand for a range of severe diseases and disorders, it has become clear that payors need a different model for assessing the value of these innovative treatments. Existing models are intended to evaluate traditional pharmaceuticals, which are often administered over a long period – sometimes throughout a patient's lifetime – to alleviate the symptoms of a disease. These models fail to adequately capture the full value of regenerative medicines and advanced therapies, which can provide significant increases in quality of life and in productivity for patients, their family caregivers and overburdened health care systems and society.
As this sector continues to grow, it is vital that payors, policymakers, and other stakeholders adopt new frameworks for value assessment, which reflect the significant benefits regenerative medicines and advance therapies deliver for the overall healthcare ecosystem. As the leading international advocacy organization and the voice of the sector, the Alliance for Regenerative Medicine (ARM) is committed to advocating for the broader adoption of new value-based frameworks.
A Transformational Therapy Value Model for Rare Blood Diseases
This past January, ARM published a new study on the potential for regenerative medicines and advanced therapies to provide medium- to long-term cost savings to society. Performed by the Marwood Institute, this study uses a first-of-its-kind "Transformational Therapy Value Model" (TVM) to demonstrate the benefits of durable therapies for three rare blood diseases: multiple myeloma, sickle cell disease, and hemophilia A.
These diseases are cumulatively projected to cost the US $163 billion – per year – by 2029. But there are currently late-stage innovative product candidates in development to treat each of them, with likely approvals in the short- to medium-term. Looking at a ten-year time frame – the same as used by the Congressional Budget Office when evaluating policy decisions – the study found that a durable therapy for each of these indications could result in aggregate cost-savings of $33 billion by 2029. These savings could begin to be realized in as few as five years.
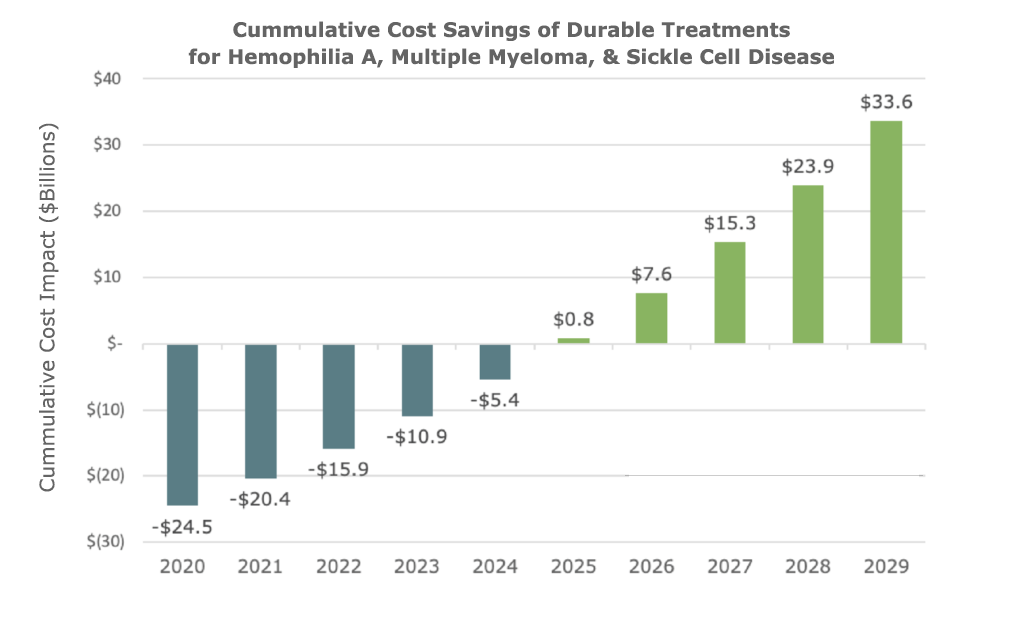
A Transformational Therapy Value Model for Rare Blood Diseases, 2020.
Hemophilia A
There are 20,000 patients in the US with hemophilia A, a genetic disorder resulting in a lack of blood clotting factor VIII, and the potential for severe bleeding. These patients, who typically require infusions of the missing clotting factor two to three times a week, have an average medical cost of $200,000 per year. Patients with severe cases can develop immunity to these infusions and may require more advanced and expensive treatments, potentially costing upwards of $690,000 to $753,000 per year. Compared to the standard of care, an approved cell or gene therapy for hemophilia could provide cost-savings of 23% annually by 2029.
Of the three diseases examined in this study, a durable treatment for hemophilia is likely closest to the market. In late 2019, BioMarin submitted marketing authorization applications to the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) for their gene therapy ValRox to treat hemophilia A. In clinical trials, patients who received the highest dose cohort saw their bleeding episodes drop 95% over four years. A decision from the FDA is expected next month. Several additional product candidates are also in development.
Multiple Myeloma
Multiple myeloma is a severe and rare form of blood cancer, with a painfully high fatality rate. About 25% of patients die within the first three years of diagnosis, and the mean survival following diagnosis is only five years. Approximately 170,000 people in the U.S. suffer from multiple myeloma, with an estimated 35,000 new cases diagnoses each year. These patients, who often require daily treatment, average $280,000 per year in medical costs. The high cost of care is driven in part by novel and expensive immunotherapies, many of which succeed only in extending patients' lives by a short period.
In addition to high medical costs, multiple myeloma also has the most substantial loss of productivity of the three diseases studied. This is due in part to the larger patient population and the older average age of multiple myeloma patients, many of whom have adult children who must take medical leave, quit their jobs, or make other costly accommodations to care for their ailing parents.
There are currently nine regenerative medicine and advanced therapy product candidates in the pipeline to treat multiple myeloma. Many of these are chimeric antigen receptor T cell (CAR-T) therapies. Similar products, Yescarta and Kymriah, have already been approved in the US, Europe, Japan, China, Canada, and Australia to treat other forms of blood cancer. The approval of a durable regenerative medicine or advanced therapy for multiple myeloma could provide annual cost savings of 30% by 2029.
Sickle Cell Disease
A serious genetic disorder primarily affecting the Black community, sickle cell disease causes a patient's red blood cells to become misshapen and break down. There are approximately 100,000 patients in the U.S. with sickle cell disease, with about 1,600 new patients diagnosed each year.
While sickle cell disease has lower annual medical costs compared to hemophilia A and multiple myeloma (on average, $30,000 per year), it can have an immensely negative impact on patients' quality of life as well as contributing to significant losses in productivity when symptoms are not well-controlled under the standard of care. These patients can suffer from frequent and extremely painful vaso-occlusive crises in which the affected red blood cells cause a blockage in smaller blood vessels, often requiring emergency department visits or hospitalization. While sickle cell disease patients live, on average, for 45 years following diagnosis, they are at high risk for serious and potentially fatal complications.
There are currently four regenerative medicine and advanced therapy products in the pipeline with the potential to durable treat sickle cell disease. Approaches include innovative gene-editing technologies, with the first patient in the US treated with a CRISPR-based therapy for sickle cell disease just last year. The approval of a durable therapy to treat sickle cell disease could result in cost savings of 18% per year by 2029.
Staying Ahead of the Wave
Regenerative medicines and advanced therapies are no longer the stuff of science fiction. Early products to market have demonstrated profound, durable, and potentially curative benefits that are already helping thousands of patients worldwide. And as of the end of 2019, there were 1,066 clinical trials ongoing worldwide – representing a robust pipeline with the potential to address the unmet medical needs of hundreds of thousands of additional patients. The number of approved products on the market will continue to grow, with the FDA predicting that 10-20 new cell and gene therapies will be approved each year by 2025.
With a new wave of transformative therapies rushing towards us, payors, policymakers, and other stakeholders must implement the infrastructure necessary to ensure broad patient access and appropriate value-based reimbursement. In addition to the potential cost-savings illustrated in this report, these therapies can also provide profound improvements in quality of life compared to the current standard of care for patients with a diverse array of serious diseases and disorders. We must ensure patients can access these therapies as quickly as possible following approval.
In our 11-year history, ARM has been the voice of the sector, working with our 350+ members worldwide to promote the legislative, regulatory, and reimbursement initiatives required to advance transformative therapies. We believe in the continuing promise of this field – and we're ready to see that promise realized.
---
Janet Lambert is the CEO of the Alliance for Regenerative Medicine (ARM). ARM is the leading international advocacy organization dedicated to realizing the promise of regenerative medicines and advanced therapies. ARM promotes legislative, regulatory and reimbursement initiatives to advance this innovative and transformative sector, which includes cell therapies, gene therapies and tissue-based therapies. Early products to market have demonstrated profound, durable and potentially curative benefits that are already helping thousands of patients worldwide, many of whom have no other viable treatment options. Hundreds of additional product candidates contribute to a robust pipeline of potentially life-changing regenerative medicines and advanced therapies. In its 11-year history, ARM has become the voice of the sector, representing the interests of 350+ members worldwide, including small and large companies, academic research institutions, major medical centers and patient groups. To learn more about ARM or to become a member, visit http://www.alliancerm.org.
* Data on disease prevalence, incidence, mortality, treatment costs, and indirect costs sourced from the American Cancer Society, American Society of Hematology, American Journal of Hematology, American Journal of Managed Care, the Centers for Disease Control, Journal of Managed Care and Specialty Pharmacy, Journal of the American Medical Association, Journal of Medical Economics, the Mayo Clinic, the New England Journal of Medicine, the National Institutes of Health, and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. For more information, see the full report.
