Weighing The Benefits Of In-House Manufacturing For Cell And Gene Therapies
By Brent Rice, PhD, Director, Manufacturing Science and Technology and Damien Hallet Senior Director, CMC Operations and Strategic Planning at Precision BioSciences

Organizations developing cell and gene therapy product lines must carefully weigh the costs and benefits of in-house manufacturing versus contract manufacturing organizations (CMOs) for each program at each stage. CMOs offer flexibility, scalability, dedicated supply channels, and decreased internal infrastructure requirements. In-house positives include ownership of supply channels as well as greater agility in process troubleshooting and the efficient development of a corporate knowledge base that will allow for improved oversite of contractors or future in-house scale up. For complex and novel therapeutics such as allogeneic chimeric antigen receptor (CAR) T cell products, these in-house advantages are important and may emerge early in clinical development. Considering these advantages, in 2019 Precision BioSciences opened its Manufacturing Center for Advanced Therapies (MCAT), the first in-house current Good Manufacturing Process (cGMP) compliant manufacturing facility dedicated to genome-edited, “off-the-shelf” CAR T cell therapy products in the US. This is part of a larger investment by the company in its cancer immunotherapy platform and facilities expansion in North Carolina.

Precision BioSciences’ MCAT facility is housed at the BioPoint Center in Research Triangle Park, North Carolina
Unique T Cell Editing Platform and Manufacturing Process
Precision BioSciences is advancing an allogeneic CAR T cell therapy pipeline of clinical candidates that may provide valuable new treatment options for patients with cancer. Autologous CAR T products (Yescarta®, Kymriah®)1 have demonstrated clinical success in recent years, helping to pave the way for new immunotherapy treatment options, yet come with limitations such as the stress of the apheresis procedure, the risk of an unsuccessful manufacturing process, and the multi-week wait time, often in an ICU setting. Allogeneic CAR T therapies offer larger, multi-patient batches of off-the-shelf T cells from healthy donors with the potential to spare very sick patients some of the unfortunate challenges of autologous CAR T product development.
Successful manufacture of allogeneic CAR T therapies depends on a gene editing technology that maintains the integrity of the T cells and ensures routine, reliable, and consistent production of CAR T batches with the goal of generating 100 doses or more from a single leukopak. The company’s proprietary ARCUS® genome editing technology is unlike other gene editing tools (e.g. TALEN, CRISPR-Cas, Zinc Finger Nuclease) and incorporates two important features: a programmable nuclease targeting a highly specific DNA target site and a resulting DNA break that leaves clearly recognizable “sticky ends” that promote subsequent gene modification steps, making ARCUS an ideal technology for therapeutic use.
In Precision BioSciences’ allogeneic CAR T manufacturing process, a transfected mRNA encoding the small (~360 aa) ARCUS nuclease creates a specific double-stranded DNA break in a 22 base pair recognition site within the TRAC (TCR alpha constant) locus of the T cells. A recombinant adeno-associated virus (AAV) vector is then used to deliver a transgene encoding the antigen-specific CAR, which integrates into the genomic TRAC locus as part of the gene editing process. This results in the site-specific insertion of the gene encoding the CAR into the endogenous gene encoding the TCR alpha subunit. Thus, in a single step, the allogeneic T cells are rendered incapable of inducing graft vs. host disease, or GvHD, (because they no longer express a T cell receptor) and can recognize and kill antigen-expressing tumor cells (because they express a CAR). The CAR construct encoded by the AAV vector is a defining difference between Precision BioSciences’ allogeneic CAR T clinical candidates and determines the therapeutic target for each (e.g. anti-CD19 for non-Hodgkin lymphoma, anti-BCMA for multiple myeloma).
The CAR T platform requires three critical manufacturing streams for the three following drug substances, all of which can be produced at MCAT: allogeneic CAR T cells, messenger RNA (including formulations development), and AAV vectors. The mRNA and AAV vectors also play central roles in Precision BioSciences’ pre-clinical in vivo gene correction programs, which also use ARCUS genome editing technology.
Integrated, In-House Manufacturing Compliments CMO Support
Despite cost, contracts, and schedules, CMOs fill inevitable voids of in-house manufacturing and also offer valuable manufacturing redundancy for late-stage development and commercial phases. However, due to the diverse nature of cell and gene therapies and the absence of a true manufacturing platform, CMOs may struggle to offer turnkey solutions for complex cell therapies as they do for conventional biologics (e.g. antibodies), where platforms are well established. While a cell and gene therapy CMO may be prepared with the knowledge of common technologies and multi-purpose equipment, significant subject matter expertise is necessary to evaluate and respond to manufacturing needs. This is particularly true if the CMO has not been involved in the development and definition of the manufacturing process. At the same time, information generated by the CMO must be relayed efficiently and securely to the in-house development and manufacturing team to ensure problems are rapidly addressed and opportunities to improve the process are recognized.
At MCAT, easy access to subject matter experts constitutes a strategic manufacturing advantage, and this ease of access has proven beneficial in more recent times of COVID-19. MCAT’s design was intentionally flexible, keeping in mind the breadth of manufacturing needs, complete with four large and flexible Grade B/C suites that can support everything from air handling for AAV to multiple high throughput CAR T lines.
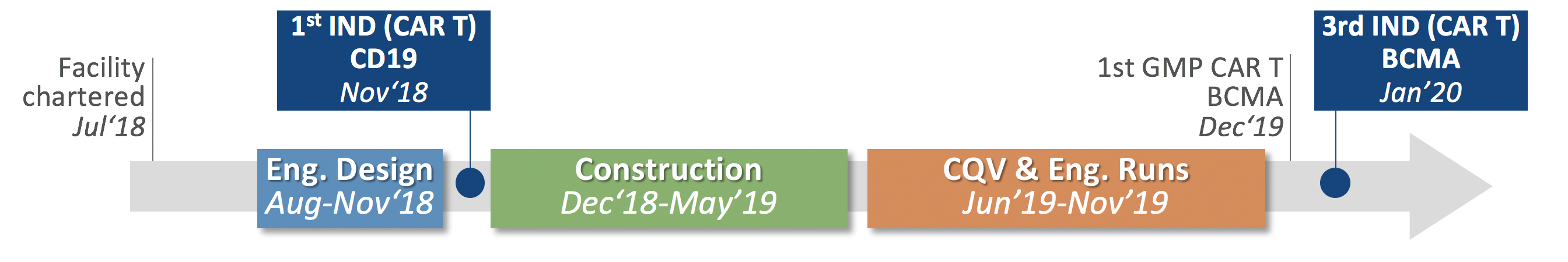
Timeline for Construction and Commissioning and Current Use of the MCAT Facility
The charter to build MCAT came prior to the approval of the company’s first IND (PBCAR0191 for the treatment of NHL and ALL) in 2018. By June 2020, Precision BioSciences initiated its third allogeneic CAR T clinical trial, a Phase 1/2a clinical trial of PBCAR269A for multiple myeloma using clinical trial material exclusively developed at MCAT. It is early but increasing advantages with in-house manufacturing program are evident and have contributed to important process optimization and improved internal expertise in producing fit, functional allogeneic CAR T cells.
Figure 1: Timeline for the MCAT construction and cGMP operational readiness
---
(1)All product names, logos, and brands are property of their respective owners. All company, product and service names used in this article are for identification purposes only.
