Using Slot Management To Bridge The Gap Between Capacity And CGTs
By Zachary Golden, Manager & Deloitte NextGen Therapies Value Chain Innovation Leader, Deloitte Consulting LLP

Supporting Authors: Hussain Mooraj, Principal & Deloitte NextGen Therapies Practice Leader, Deloitte Consulting LLP; Rajesh Singh, Managing Director & Deloitte NextGen Therapies Technology Leader, Deloitte Consulting LLP
In the fast-paced world of cell and gene therapies, we're not just fighting diseases, we're racing against time. These cutting-edge treatments hold the promise of curing conditions once deemed incurable. Yet, despite their potential, significant challenges stand in the way: skyrocketing costs for patients, high manufacturing expenses, lengthy turnaround times, and a lack of accessibility that leaves many patients waiting in uncertainty.
At the heart of these issues lies a crucial component: Slot Management. Understanding and optimizing this process isn't just beneficial, it's essential for transforming hopes into realities.
The Burning Problem: Barriers to Accessible Therapies
Despite scientific breakthroughs and remarkable progress, the cell and gene therapy industry continues to grapple with significant challenges:
- High Prices for Patients
- Exorbitant Manufacturing Costs
- Long Turnaround Times
- Limited Access
The Legacy Framework: Advanced Planning & Scheduling (APS) in the Era Before CGT
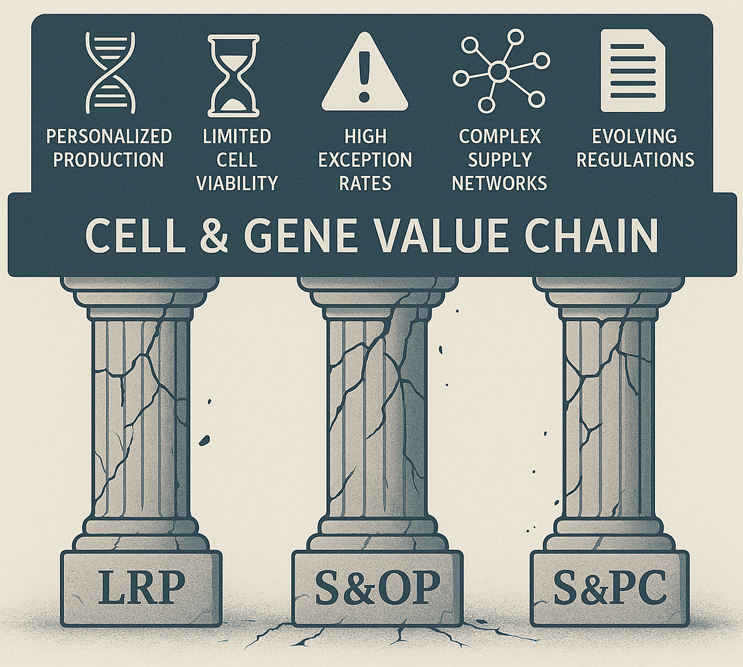
Typically, for traditional small and large molecule pharmaceutical manufacturing, the APS lifecycle that underpins the supply chain is made up of three pillars:
- Long Range, Strategic Planning (LRP): We set out the roadmap for the future (~5 years), planning our portfolio, forecasting demand, determining necessary capacity, and setting long-term objectives that guide our strategic decisions.
- Sales & Operations Planning (S&OP): This phase harmonizes our strategic goals with operational capabilities over the medium term (12-24 months), aligning Clinical, Commercial, and CMC demands with what we can realistically support.
- Scheduling & Production Control (S&PC): Here, we leverage the plan coming out of our S&OP cycle and fine-tune daily operations within each supply node, optimizing schedules, assigning resources, and tactically adjusting based on constraints and deviations.
Built for Batches, Not for Patients: Why Traditional APS Fails in CGT
Unlike traditional biopharmaceutical models, cell and gene therapies typically operate on a “make to order” or “differentiate to order” basis rather than a "make to stock" model. This fundamental difference introduces unique complexities:
- Personalized Production: Both in the case of autologous and allogeneic ex vivo therapies, the drug product is often tailored to an individual patient. In stark contrast with traditional pharmaceuticals, in which massive batches can directly be manufactured following S&OP and orders come only once a finished good is sitting on a shelf, cell and gene therapies often have “n of 1” batches. This drives a need for 1-to-1 supply and demand pegging to ensure precise allocation of supply and execution of activities.
- Limited Cell Viability: Patient cells, often the key raw material for these cutting-edge therapies, have highly limited “shelf lives” measured in hours rather than days, weeks, or months unless cryopreserved. An accidental double-booking, causing one patient’s cells to become no longer viable after waiting an extra day, very well might be the difference between life and death in the case of a cancer patient. Confidence in the schedule, therefore, is table-stakes. And the need for real-time adjustments to production schedules is critical to accommodate both demand and supply exceptions.
- Demand Variability and High Exception Rates: Biopharmaceutical organizations, once many layers removed from the patients they serve, are now directly impacted by the typical variability in a patient’s schedule. This demand variability, equating to variability in raw material supply, creates a dynamic and unpredictable production landscape. For instance, CAR-T therapies can have a demand exception rate of up to 65%, driven by patients’ need to reschedule or even cancel their collection appointments. These disruptions significantly impact a biopharmaceutical organization's ability to achieve 100% slot utilization while maintaining reliability.
- Complex Supply Networks: Biopharmaceutical organizations increasingly rely on CDMOs and suppliers who must play dual roles as both vendors and customers while scaling up this new value chain. Simply put, the CGT value chain requires tighter control over the collaboration but inherently has less. Setting and linearizing the schedule across these varying nodes is critical to achieving quality patient outcomes.
- Evolving Regulatory Landscape: With regulations constantly evolving across this complex network, organizations must remain vigilant in maintaining and enforcing business rules, monitoring changes, and adjusting approvals accordingly.
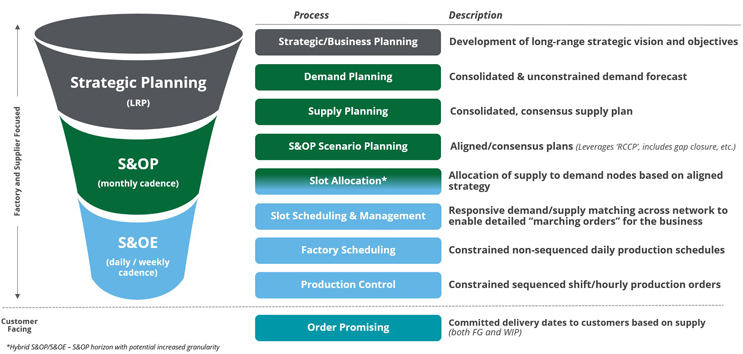
Slot Management is the Fourth Pillar & “Secret Ingredient” for CGT
Slot Management – the fourth pillar of APS sitting between Sales & Operations Planning and Scheduling & Production Control – is the executional brain of the supply network over the organization’s tactical horizon (typically between 0-24 weeks). As such, it is responsible for creating an optimally linearized schedule across the supply chain, thus enabling all parties to march to the same drumbeat across sites, countries, and continents.
Slot Management does this by enabling three sets of capabilities: Slot Allocation, Slot Booking, and Exception Management.
- Slot Allocation: For organizations who are supply constrained, the process of allocating – or walling off supply – allows for a more equitable, controlled consumption by various parties. To conduct Slot Allocation, we leverage a set of business rules, approvals, and actual tactical horizon supply availability to break the monthly S&OP consensus plan into daily or weekly buckets of walled off supply for patient (Commercial & Clinical) and non-patient (CMC, tech transfer) demands.
- Slot Booking: By gaining visibility to supply availability across the entire network, we enable real-time supply & demand matching, or booking of slots, by specific elements of demand. Similar to how airlines enable booking of seats on specific flights, Slot Management can help ensure a treatment center books only the slots which are available for their patient, block others from booking an already assigned slot, and we have even seen some manufacturers over-book to minimize risk of empty slots due to unexpected changes (cancelations, reschedules, etc.).
- Exception Management: Given Slot Management acts as the linchpin of the end-to-end supply chain schedule, it is crucial that it have visibility to and the ability to manage exceptions. Exceptions can take two forms:
- Demand Exceptions: Deviations to the plan coming from the “demand” side of the equation such as collection reschedules, order cancelations, and order holds.
- Supply Exceptions: Deviations to the plan coming from the “supply” side of the equation such as capacity reductions, missing materials, or delayed shipments.
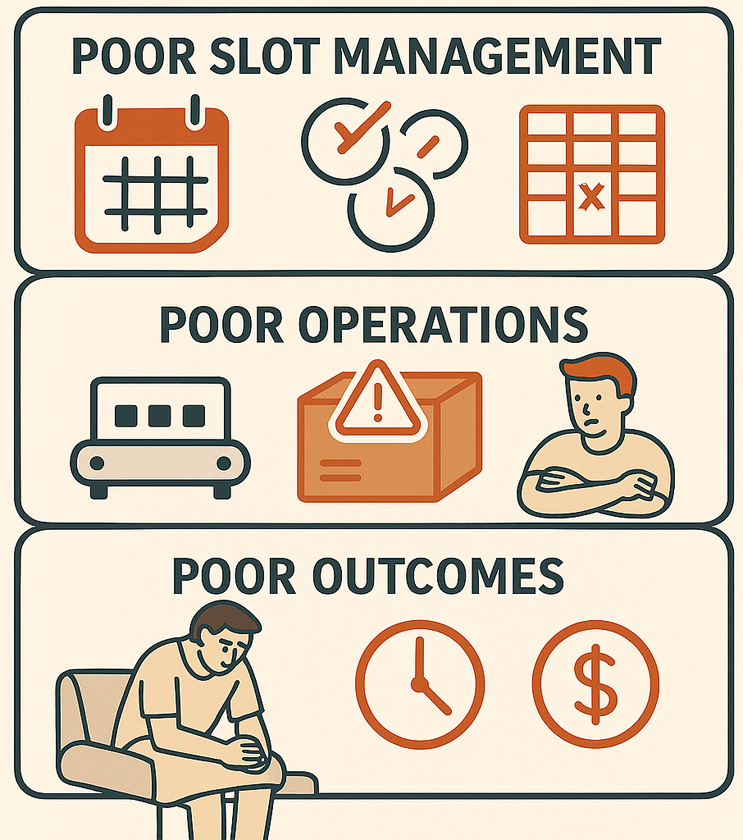
Poor Slot Management = Poor Operations = Poor Outcomes
Many organizations early on in clinical trials start managing their slots in a shared Excel tracker or manually uploading them into a QTC portal itself. They learn quickly, however, that as volumes grow, this practice rapidly breaks. Our direct experience supporting a commercialized autologous cell therapy manufacturer further illuminates these challenges. As their therapies moved from initial launch toward true scale, the senior supply chain leader voiced mounting concerns about what lay on the other side of the organization’s “inflection point.”
With volumes on the cusp of nearly tripling, the supply network footprint poised to more than double, and market coverage set to expand rapidly, the leader was candid: without a robust Slot Management platform, the wheels would begin to come off. This wasn’t a challenge that more staff could fix—no amount of headcount could solve it.
The complexity and velocity of change at this stage made clear that ad hoc or legacy scheduling approaches would be rapidly outpaced, and the repercussions of poor Slot Management would ripple across the entire value chain.
- Underutilized Capacity & Over-Bookings: Lack of real-time visibility to slot availability drives risk to both under-consumption and over-consumption of slots. Empty slots mean higher COGM, wasted opportunities to produce therapies, and poorer patient outcomes. Arguably worse than missing slots, telling a patient we can manufacture their therapy when we can’t.
- Increased Wait Times: Inefficient scheduling, such as a double-booking requiring a patient reschedule, leads to treatment delays, forcing patients to wait longer for treatment and causing further frustration.
- Cost Penalties: Many organizations working with CDMOs work consumption-based language into their agreements. Failure to meet pre-specified levels of slot utilization may result in penalties or more expensive rates per dose.
- Degraded Brand Reputation: Given high costs, long turnaround times, and low reliability, organizations who cannot manage their slots effectively consistently see lowered brand confidence and interest in the therapies altogether from the market.
Consider a patient waiting for a critical cancer therapy. Each day counts, yet due to suboptimal Slot Management, their treatment is delayed. Not only does this prolong their suffering, but it also adds emotional and financial strain.
Making Every Slot Count: The Impact of a Robust Slot Solution
Slot Management isn't just a step in the process, it's the linchpin that can alleviate these pressing issues by:
- Real-Time Supply/Demand Matching: Real-time visibility to end-to-end network supply availability enables faster booking, automated and dynamic exception management, increased customer trust, and improved brand perception.
- Maximizing Resource Utilization: By enabling 100% slot utilization, we ensure that every available production slot is filled and never double-booked, reducing waste and improving patient outcomes.
- Reducing Costs: Streamlined operations decrease the COGM, which can translate into lower prices for patients and a more sustainable model for manufacturers. Organizations who have implemented cutting edge Slot Management solutions have, on average, increased utilization upwards of 15%.
- Optimized Cost-to-Serve: Incorporation of configurable business rules enables organizations to balance service levels and cost by allowing them to set optimal flow of each order through the value chain.
- Enhancing Access: Efficient slot allocation expands our ability to serve more patients more fairly, breaking down barriers to access.
- Reduced Barriers to Launch: Slot visibility across many types of constraints enables organizations to support both existing and new products across many modalities and platforms in both Clinical and Commercial phases.
Consider, for example, that same cell therapy manufacturer. After partnering to implement a best-in-class slot management capability—complete with automated, optimization-based schedule recommendations—the organization now finds itself confidently on the other side of the “inflection point.” Utilization rates have significantly improved, turnaround times are consistently solid, and, with this foundation firmly in place, the business is eagerly looking to the future. There are already plans to expand the slot management capability to support a new asset, further strengthening operational agility and extending impact across a broader therapeutic portfolio.
Where Intelligence Meets Urgency: Tech’s Role in Slot Management
So, how do we push Slot Management to its full potential? The answer lies in harnessing advanced technologies:
- Simulation Modeling: Creating digital twins of the value chain allows us to simulate and analyze different scenarios without impacting actual operations. We can experiment with variables like demand fluctuations, equipment downtime, or supply chain disruptions to develop robust strategies.
- Agentic AI: By employing Agentic AI, Slot Management can be taken to the next level, allowing your organization to scale with minimal impact to headcount. This form of AI can recommend or even autonomously make decisions in real-time. Agents can help organizations level-load their supply network, and flex slot allocations while minimizing human intervention.
- Multi-Objective Optimization Engines: By considering multiple goals, such as maximizing slot utilization while minimizing costs and turnaround times, we can find the optimal balance that satisfies critical metrics. This comprehensive approach ensures we're not improving one aspect at the expense of another.
- Advanced Analytics: Deploying predictive analytics helps in forecasting trends and making proactive adjustments. Data-driven insights enable us to anticipate challenges before they escalate into problems.
By integrating these technologies into Slot Management, we can make therapies more affordable, accelerate time-to-treatment, lower manufacturing costs, and expand access.
Every Slot, Every Patient: What We’ve Learned and What’s Next
Slot Management stands as the pivotal solution to some of the most pressing challenges in cell and gene therapy today. By honing this critical process and implementing advanced technology-driven Slot Management solutions, we not only improve operations but, more importantly, bring hope closer to patients worldwide.
Successfully implementing Slot Management capabilities in the personalized medicine space has provided us with invaluable insights. Here are some key lessons learned:
- One Size Doesn’t Fit All: Slot Management is not a one-size-fits-all solution. Each organization has its own unique policies, constraints, and value chains. Tailoring the approach to fit these specifics is crucial for successful implementation.
- Importance of Change Management: Effective change management is at the heart of successfully rolling out and scaling Slot Management capabilities. Engaging stakeholders, managing expectations, and guiding teams through transitions are essential for long-term adoption and impact. Imagine a lead scheduler, who now, instead of manipulating a myriad of excel sheets can with “one-click” get a recommendation on how slots need to be re-adjusted based on a schedule change. This operational simplification captures the profound shift that comes when manual processes are replaced with intelligent automation—hours once spent on tedious tasks are suddenly freed up, underscoring the importance of thoughtful change management in driving both efficiency and acceptance across the organization.
- Architecting and Upskilling for AI: Integrating AI into Slot Management necessitates careful architectural planning and a focus on upskilling the workforce. Employees need to be trained to work alongside AI systems, significantly enhancing decision-making and operational efficiency.
Many organizations across the cell and gene therapy landscape are increasingly aware of these complexities. There is a growing recognition of the critical need to address them with tailored Slot Management approaches that meet each enterprise where it is—accounting for unique policies, value chains, and operational requirements. By harnessing advanced technologies such as AI, multi-objective optimization, and simulation modeling, companies can design and implement solutions finely tuned to their specific needs. This strategic alignment can enable organizations to not only keep pace in a rapidly evolving industry, but it can also deliver better outcomes for patients.
This publication contains general information only and Deloitte is not, by means of this publication, rendering accounting, business, financial, investment, legal, tax, or other professional advice or services. This publication is not a substitute for such professional advice or services, nor should it be used as a basis for any decision or action that may affect your business. Before making any decision or taking any action that may affect your business, you should consult a qualified professional advisor.
Deloitte shall not be responsible for any loss sustained by any person who relies on this publication.
About The Authors
Zachary Golden
 Zach is a Manager in Deloitte’s NextGen Therapy practice, where he serves as the AI & Value Chain Innovation Lead. He drives transformation at the intersection of operational excellence and digital innovation, shaping the firm’s perspective on Advanced Planning & Scheduling, Artificial Intelligence, and Orchestration for Cell & Gene Therapies.
Zach is a Manager in Deloitte’s NextGen Therapy practice, where he serves as the AI & Value Chain Innovation Lead. He drives transformation at the intersection of operational excellence and digital innovation, shaping the firm’s perspective on Advanced Planning & Scheduling, Artificial Intelligence, and Orchestration for Cell & Gene Therapies.
With over 8 years of industry and consulting experience in Life Sciences, Zach brings deep expertise across planning and scheduling, manufacturing, global logistics, chain of identity/chain of custody (CoI/CoC), operational control tower implementations, real-time track & trace, and field investigation and action management.
Hussain Mooraj
 Hussain is a Principal in Deloitte’s Life Sciences Enterprise Performance practice. He leads Deloitte’s Global NextGen Therapy practice and the Clinical Trial Supply Chain offerings. He brings 25+ years of experience in sales & marketing, supply chain, manufacturing, and strategy consulting. He has led engagements in healthcare value chain strategy, global planning and S&OP, ERP roll outs, clinical supplies, outsourced manufacturing, custom technology solutions, global logistics and control tower projects, and transformation of traditional supply chains to digital supply networks.
Hussain is a Principal in Deloitte’s Life Sciences Enterprise Performance practice. He leads Deloitte’s Global NextGen Therapy practice and the Clinical Trial Supply Chain offerings. He brings 25+ years of experience in sales & marketing, supply chain, manufacturing, and strategy consulting. He has led engagements in healthcare value chain strategy, global planning and S&OP, ERP roll outs, clinical supplies, outsourced manufacturing, custom technology solutions, global logistics and control tower projects, and transformation of traditional supply chains to digital supply networks.
Rajesh Singh
 Rajesh is a NJ based Managing Director in Deloitte’s Life Sciences Digital Supply Chain Technology practice with over 18 years of large-scale global IT Strategy, System Architecture & Implementation and Program Management experience. He also leads the technology stream of Deloitte’s NextGen Therapy offering. He specifically focusses on Pharma / Biotech and NextGen Therapy companies, assisting them with system architectures, platform selection (incl. ERP), developing early IT strategies, and technology architectures from product launch to future growth.
Rajesh is a NJ based Managing Director in Deloitte’s Life Sciences Digital Supply Chain Technology practice with over 18 years of large-scale global IT Strategy, System Architecture & Implementation and Program Management experience. He also leads the technology stream of Deloitte’s NextGen Therapy offering. He specifically focusses on Pharma / Biotech and NextGen Therapy companies, assisting them with system architectures, platform selection (incl. ERP), developing early IT strategies, and technology architectures from product launch to future growth.
About Deloitte
Deloitte refers to one or more of Deloitte Touche Tohmatsu Limited, a UK private company limited by guarantee (“DTTL”), its network of member firms, and their related entities. DTTL and each of its member firms are legally separate and independent entities. DTTL (also referred to as “Deloitte Global”) does not provide services to clients. In the United States, Deloitte refers to one or more of the US member firms of DTTL, their related entities that operate using the “Deloitte” name in the United States and their respective affiliates. Certain services may not be available to attest clients under the rules and regulations of public accounting. Please see www.deloitte.com/about to learn more about our global network of member firms.
Copyright © 2025 Deloitte Development LLC. All rights reserved.
