Tips For Filing Regenerative Medicine Patents
By April Wurster and Christopher Bright, Snell & Wilmer

Intellectual property protection is a key challenge for the regenerative medicine market. Specifically, the patent eligibility requirement of U.S. patent law creates obstacles for this sector that traditional biotech companies do not face as frequently. This article examines common regenerative medicine claims for cell and gene therapies as well as biologics that are likely patent ineligible and provides potential claim amendments and other strategies for converting them into claims that are likely patent eligible.
Overview Of Section 101
35 U.S.C.S. § 101 defines what subject matter is eligible for patent protection in the United States. The U.S. Patent and Trademark Office uses two criteria for subject matter eligibility: (1) the claimed invention must fall into one of four statutory categories: processes, machines, manufactures, and compositions of matter, and (2) the claimed invention must generally not be directed to a judicial exception. Judicial exceptions include abstract ideas, laws of nature, and natural phenomenon. If the claims are not directed to a judicial exception, the claim qualifies as patentable subject matter under § 101. If the claims are directed to a judicial exception, the Patent Office will next determine whether the claim recites additional elements that amount to significantly more than the judicial exception. This is the so-called hunt for an “inventive concept.” Due to the subjectivity of the search for an inventive concept and the difficulty of rebutting an examiner's assertion that a technique is routine and well known in the art, it is often advantageous, when possible, to write or amend claims so they are not directed to a judicial exception. This article looks at potential ways to write or amend claims to recite patent eligible subject matter for regenerative medicine.
Section 101 Applied To Regenerative Medicine
1. Composition Claims
A typical regenerative medicine composition claim might read: an isolated human pacemaker cell. Human pacemaker cells are naturally occurring. As such, a claim to an isolated human pacemaker cell would likely not be patent-allowable under the product-of-nature judicial exception.
However, with careful patent drafting this claim might be amended such that this patent ineligible claim becomes patent eligible. For example, cell populations that incorporate artificial modifications would more likely be held patent eligible.
The following table lists different claim types, whether they are likely patent eligible or not and potential amendments that may transform the claim into likely patent eligible subject matter:
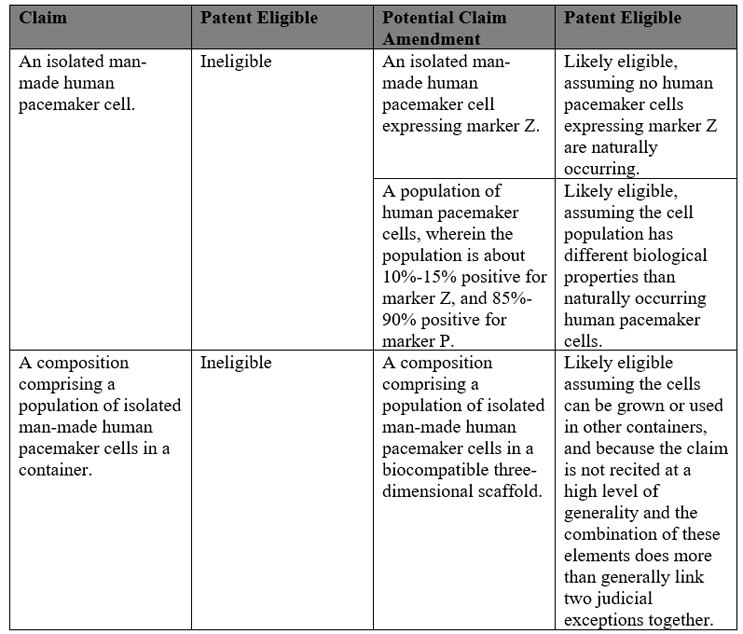
Specific examples of composition claims that would be more likely to be found patent eligible include:
- Cell populations:
- An erythroid cell comprising an exogenous polypeptide
- Growth or differentiation media
- A cell culture medium comprising media to maintain the cell population in an undifferentiated state
- A cell culture medium comprising media to differentiate the cell population into a new cell population
- Biopharmaceutical compositions
- A therapeutic composition comprising cells derived from adipose tissue cells that express a marker not present in nature
- A pharmaceutically acceptable composition comprising aggregate stem cells
- Cells comprising conjugates or fusion proteins in which cells are combined with other molecules to produce a non-natural cell
2. Method Claims
Method claims often fall into three categories: (1) method of treatment claims, (2) method of producing claims, and (3) diagnostic claims.
Method of Treatment Claims
A typical method of treatment claim for a regenerative medicine company might read: a method of treating a patient for a disease comprising administering an effective number of cells expressing a liver marker to restore liver function.
Claims “directed to a specific method of treatment for specific patients using a specific compound at specific doses to achieve a specific outcome” have been found patent eligible by the Federal Circuit. Endo Pharmaceuticals Inc. v. Teva Pharmaceuticals USA, Inc., No. 2017-1240 (Fed. Cir. 2019).
Method of Producing Claims
A typical method of producing claim for a regenerative medicine company might read: a method for producing a liver cell comprising contacting an iPSC with a compound, thereby generating a cell population expressing liver markers.
Specific examples of method of producing claims found patent eligible include:
- A method for producing a gene-edited cell
- A method for reprogramming a cell
- A method for producing a gene-edited, reprogrammed cell
- A method for producing a progenitor cell in vitro
Generally speaking, claims directed to a specific method of differentiating a cell population using a specific compound are typically patent eligible.
Diagnostic Claims
While method of treatment claims and methods of production claims are often found patent eligible, diagnostic claims are often found ineligible because they are merely directed to the observation, detection, or diagnosis of medical phenomena using routine or conventional techniques. For example, a method of detecting paternally inherited cell-free fetal DNA in a maternal blood sample was found patent ineligible. Ariosa Diagnostics, Inc. v. Sequenom, Inc., Slip Op. 2014-1139, 2014-114 (Fed. Cir. 2015). This approach to diagnostic claims has been affirmed in Caredx, Inc. v. Natera, Inc., Slip Op. 2022-1027, 2022-1028 (Fed. Cir. 2022) (“Here, as in Ariosa, the claims boil down to collecting a bodily sample, analyzing the cfDNA using conventional techniques, including PCR, identifying naturally occurring DNA from the donor organ, and then using the natural correlation between heightened cfDNA levels and transplant health to identify a potential rejection, none of which was inventive. The claims here are equally as ineligible as those in Ariosa.”).
How can such a claim be amended to be patent eligible? A method of diagnosis directed to actions instead of correlations is more likely to be patent eligible. For example, a claim reciting a method of diagnosing a patient comprising extracting DNA, producing DNA fragments by size discrimination and selectively removing DNA fragments larger than a particular size, and analyzing the DNA fragments, wherein the presence of DNA fragments larger than a particular size indicates a disease is present, is more likely to be patent eligible.
Patent Eligibility In Litigation
While patent owners may want to avoid arguments related to “inventive concept” during prosecution, taking such positions in litigation of regenerative medicine patents may be helpful. Recently, the Federal Circuit held that the issue of whether claim limitations recite routine or conventional subject matter – rather than an “inventive concept” – may raise questions of fact that preclude resolution early in the case. Berkheimer v. HP Inc., 881 F.3d 1360, 1367 (Fed. Cir. 2018). As such, a patent owner may, where possible, want to consider alleging nonroutine or unconventional aspects of the claimed subject matter in its pleading to help avoid an early adverse decision on patent eligibility.
Conclusion
Section 101 is a threshold test for patent eligibility. Regenerative medicine raises special considerations for patent eligibility. With careful claim drafting, applicants for regenerative medicine patent claims may be able to improve their chances of obtaining and defending their patents against patent eligibility challenges. For more information, the Patent Office has created specific patent eligibility guidelines for regenerative medicine companies, which can be found here.
 About The Authors:
About The Authors:
April Wurster is counsel in the San Diego office of Snell & Wilmer. She focuses her practice on intellectual property and life science, and has extensive experience with patent protection and trademark protection.
 Christopher Bright is a partner in the Orange County and San Diego offices of Snell & Wilmer. He has more than 20 years of experience litigating patent cases across the United States, as well as providing counseling for global patent licensing negotiations and patent due diligence for mergers and acquisitions. Bright has extensive scientific experience and has skillfully tried numerous patent cases in U.S. federal district courts and before the International Trade Commission.
Christopher Bright is a partner in the Orange County and San Diego offices of Snell & Wilmer. He has more than 20 years of experience litigating patent cases across the United States, as well as providing counseling for global patent licensing negotiations and patent due diligence for mergers and acquisitions. Bright has extensive scientific experience and has skillfully tried numerous patent cases in U.S. federal district courts and before the International Trade Commission.
