The Moving Regulatory Landscape For Gene Therapy Trials In EU: Part 1
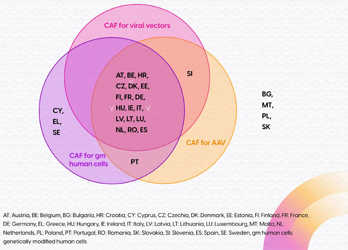
The CTR mandates the use of the CTIS for CTA submissions in the EU, but doesn't address GMO applications for gene therapy studies. This omission is problematic as GMO application requirements and procedures vary significantly across Member States. To mitigate these challenges, specific CAFs for GTMPs have been developed. These CAFs aim to simplify the GMO application process by providing a standardized framework for environmental risk assessment and reducing the information required. However, their adoption is not universal among EU Member States.
Additionally, the Netherlands has implemented a more advanced approach, where clinical sites act as GMO license holders, further streamlining the process. While the CAFs offer a degree of harmonization, further advancements are needed to fully address the complexities of GMO applications for GTMPs in the EU. The proposed new pharmaceutical legislation presents an opportunity for further improvements in this area.
Learn more by reading the full article below.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
