The Importance Of Feasibility Assessments In The Standards Development Process
By Catherine B. Zander, Ph.D.; Scientific Program Manager at the Standards Coordinating Body

The development and production of regenerative medicine advanced therapies faces substantial obstacles including fragmentation of knowledge, insufficient communication and coordination among developers and other stakeholders, and unpredictable advancement of innovation. Creation and application of standards in this industry can help address these challenges (Figure 1). Accelerating the adoption of standards relating to best practices in scientific protocols, product testing, product quality and performance specifications will also help accelerate innovation, encourage precompetitive collaboration, knowledge sharing, and help facilitate the regulatory review process. In turn, potentially reducing the cost to patients. The use of standards also increases the safety and reliability of new therapies, helping to build public trust and support.
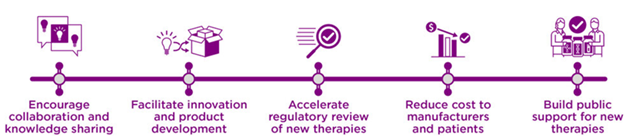
Figure 1. Benefits of regenerative medicine standards.
Regenerative medicine advanced therapies have relatively few existing standards compared with more mature therapeutic modalities. As such, researchers, drug developers and manufacturers are often left to solve many frequently occurring but complex challenges of clinical translation and scaling of commercial products independently. Furthermore, regenerative medicine products are often highly personalized and consist of dynamic living cells, which creates unusual difficulties not faced by other therapeutic product areas with establishing common practices for comparing test results and ensuring consistent product quality and safety.
The Standards Coordinating Body (SCB) coordinates the accelerated development of standards and best practices to address the rapidly evolving needs of these industries. The SCB occupies a unique niche within the regenerative medicine ecosystem, as it is a nonprofit with no vested interest in a particular scientific, commercial, clinical, or policy approach. SCB accomplishes it mission by connecting and educating the regenerative medicine advanced therapy community to the standards development process.
Identification of Needed Standards
To support the regenerative medicine advanced therapy community in advancing new efficient, and scalable practices and reduce the burden on companies seeking regulatory approval for their products, SCB interviews stakeholders to identify gaps in existing standard coverage and potential opportunities where standards could yield significant benefits. The needed standards are listed in our publication entitled: “Community Perspectives: Needed Standards in Regenerative Medicine” https://www.standardscoordinatingbody.org/needed.
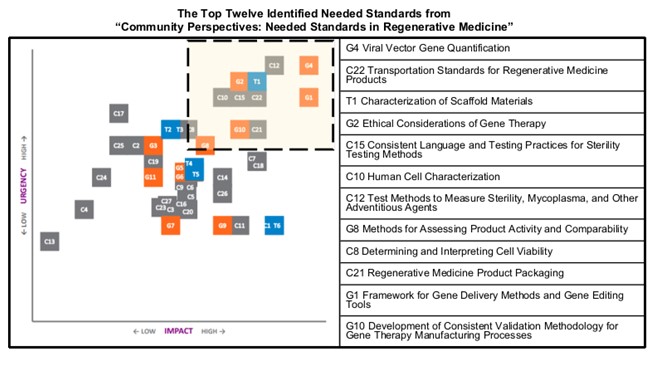
Figure 2 Top 12 Identified Standards Needs
In this living document, which is revised periodically, SCB collects information from regenerative medicine stakeholders to identify, analyze, and summarize needed standards. The document currently outlines more than 30 areas where standards are needed. These areas are prioritized and categorized by sector and application area, from bioprocessing to clinical trials. Figure 2 shows all 30 of the standards in the perspectives report graphed according to their reported impact and urgency and are labeled by sector (“G” indicated gene therapy; “C” cell therapy; “T” tissue engineering). Based upon input from stakeholders the twelve most impactful and urgent areas of need have been identified (Figure 2, yellow box). The urgency and impactful ratings are revisited annually by SCB through the canvassing of regenerative medicine stakeholders. With the progress of cell and gene therapeutic products, it should be no surprise that areas, such as determining and interpreting cell viability and gene delivery methods/gene editing tools were identified as some of the most urgent and impactful.
While the Need Standards Report identifies urgency and impact of needed standards, it does not indicate whether a potential standard is ready to be further developed and published. SCB takes these data and uses an objective and systematic approach to determine the feasibility of a needed standards area. Consequentially, this approach will dictate what area of standards needs will be prioritized for standards development activities by SCB.
Feasibility Assessments
A feasibility assessment is the systematic analysis and thoughtful evaluation of a potential standard’s benefits to the field as well as anticipated impediments to its scientific maturity, projected economic impact, and other relevant factors. To manage these evaluations, the issues comprising feasibility have been sorted into factors. Tier 1 factors are the essential issues to consider for a standard’s successful adoption; they include technical, economic, and operational factors. Tier 2 factors are issues that focus mainly on the development process. Although they are still essential for a standard’s success, they are only evaluated if a standard is likely to be adopted, as assessed in Tier 1. Tier 2 factors include legal issues, resources, and schedule. A schematic of SCB’s feasibility process is shown in Figure 3.
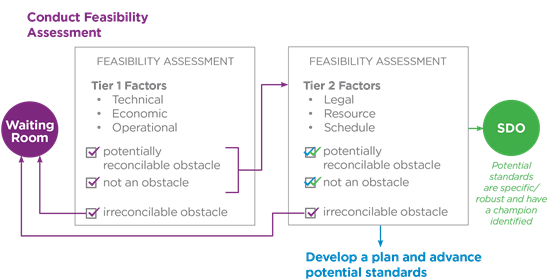
Figure 3. Schematic of SCB’s feasibility process
Figure 3 shows the paths a potential standard may make through the feasibility process.
- First, an assessment of tier 1 factors is conducted.
- If there are irreconcilable obstacles, the potential standard is moved to the “waiting room” where SCB will monitor the area and conduct another feasibility assessment if there are any major changes to the Tier 1 factors.
- If there are no Tier 1 irreconcilable obstacles, the potential standard is then assessed for Tier 2 factors.
- If there are irreconcilable obstacles, it is moved to the “waiting room”.
- If both Tier 1 and Tier 2 are found to not to have irreconcilable obstacles the potential standard will either be developed by SCB so it can eventually be advanced to a Standards Development Organization (SDO).
- If the potential standard is already well developed or has a champion at an SDO, the potential standard may move to an SDO without any additional development by SCB.
With the tiers in mind, a feasibility group is established by recruiting volunteers who are working in the field or with the relevant technology and reflect the diversity of expertise and viewpoints to effectively assess the two tiers. SCB recruits through its stakeholder networks and continually adds expert volunteers to our network. (To join a current or future feasibility group, please contact SCB either through our webpage or by emailing us at Admin@regenmedscb.org.)
Once the feasibility group is formed, a series of discussions are guided by the SCB scientific program managers using a series of questions to assess Tier 1 and Tier 2 feasibility factors to guide the feasibility discussion. At the conclusion of the discussions, the scientific program manager uses the collected information to rate the statements with respect to Tier 1 and 2 factors.
Examples of Tier 1 feasibility statements might include:
- “A firm can adopt the standard without a significant adverse impact on capital costs.”
- “There is sufficient evidence to support that the standard will have the desired impact/purpose.”
- “A firm can adopt the standard without significant operational changes or without requiring additional resources, technology, and operational infrastructure.”
- “The required operational, engineering, and technical expertise is unlikely to vary by company size or location, preventing differential burden.”
The scientific program manager will assign the statement a rating between 0-3; “0” indicates the statement is entirely false and “3” indicates the statement is entirely true. The statements are weighted, and once all the feasibility statements are rated, a final feasibility score is calculated. SCB will use the feasibility score to determine the next steps.
Two recent areas of standards need that were identified as urgent and impactful by the community that were published in the Needed Standards Report and have subsequently undergone feasibility assessments using the SCB process are:
- determining and interpreting cell viability.
- gene delivery methods/gene editing tools. Cell Viability was found to have no insurmountable issues in any Tier 1 or Tier 2 factors, and will enter the standards development process. In contrast, the subtopic of “off target effects,” within gene delivery methods/gene editing tools was found to have insurmountable technical factors, as the field is not yet in agreement on best practices and methods. Therefore, it will not be moving into the formal standards development process at this time. However, to help address this area of need, SCB is forming a working group to draft a white paper that will define the common challenges and share the solutions some companies have developed internally. The input gathered in development of the white paper will help facilitate the creation of a standard that will be better tailored to real-world needs and best practices and is likely to be embraced by the community.
This SCB process helps promote the efficient use of limited resources across the regenerative medicine community. Assessment results are shared publicly and can help demonstrate the value of developing a specific standard. It is essential to note, however, that it is as important to identify which standards needs are not ready to enter the development process, as it is to identify the ones that are. Until these high priority and urgent areas of standards needs can be addressed, they will continue to be unresolved problems and gaps that threaten the success and advancement of regenerative medicine therapies. A public feasibility assessment to highlight the issues can help to move them forward until they are ready to be developed into a standard. For both standard needs that are feasible to develop into standards, and those that are not, the SCB process works to identify and recruit champions needed to advance the standard or area of needed standards, and to build buy-in for the standard’s eventual adoption.
Conclusion
Feasibility assessments are a key component of SCB’s strategy to accelerate standards development for regenerative medicine advanced therapies. These assessments are a systematic approach that can be objectively applied to potential standards and areas of standards needs and can identify the standards with the greatest potential to be published and used by developers and manufacturers. The public availability of the feasibility reports allow interested stakeholders, potential working group members, and champions to learn more about the challenges and opportunities. Their recruitment supports the advancement of the standards identified to require additional support, expertise, or information. Ultimately, the feasibility assessments assure that urgent and impactful potential standards are advanced as rapidly as possible to the benefit and advancement of regenerative medicine advanced therapies.
Get Involved
If you or your organization would like to learn more about SCB’s current efforts, please visit our webpage at StandardsCoordinatingBody.org. Or if you would like to be involved in any of SCB’s working groups or feasibility assessments you can either visit our webpage at StandardsCoordinatingBody.org/how-to-get-involved or contact us directly by emailing Admin@RegenmedSCB.org.
---
Catherine (Katie) B. Zander, Ph.D.
Dr. Zander is currently a scientific program manager at the Standards Coordinating Body. In this role, she works to facilitate conversations and coordinate stakeholders within the regenerative medicine community to accelerate the creation of and the use of standards for cell and gene therapies and tissue engineering. Prior to her work at SCB, Dr. Zander was the American Society of Hematology’s first AAAS Science & Technology Policy Fellow, where she worked for the U.S. House of Representatives, Committee on Energy and Commerce (Democrats). In the Committee she worked on a variety of issues ranging from drug shortages, the 21st Century Cures Act, and maternal mortality, to nuclear waste cleanup and storage and the regulation of toxic substances. Prior to the fellowship, she was a postdoctoral fellow at the University of Alabama at Birmingham’s Medical School. In X. Long Zheng, M.D., Ph.D.’s lab, Katie researched the rare blood disease, thrombotic thrombocytopenic purpura (TTP), and established a TTP patient education program in Alabama.
