The Cell and Gene Therapy Dilemma: Centralized Vs. Decentralized Manufacturing
By Sanjay Srivastava, Ph.D., Cell & Gene Therapy Lead, Life Sciences IX.0/SCO Practice; Gaurav Shah, Industry X GTM- Life Sciences; Adam Volini, Principal Director; and Quinn Civik, Senior Analyst at Accenture

We have seen several regulatory and clinical successes in cell and gene therapy (CGT), highlighted by more than 40 CGT approvals by USFDA achieved thus far1. Unfortunately, to date, access to these therapies has been limited to very few eligible patients. For example, it is estimated that less than 20 percent of eligible B-Cell Lymphoma patients are receiving CAR-T therapy in the U.S.2 There are several possible reasons for this: high therapy prices, high cost of manufacturing, CGT products not available in low-GDP countries, the inability to scale manufacturing, and the endemic slow incorporation of complex treatments in many health systems globally. But there is consensus that the main reason for limited patient access continues to be industry’s inability to make products efficiently and at scale.
Is decentralized manufacturing the solution?
The industry has recently been enraptured by the decentralized manufacturing of cell therapies, with automated instruments producing cells at the point of care (POC). The hope is that decentralized manufacturing could improve CGT access by addressing issues related to the high cost of production, scale, and distribution. Many of these arguments overlook several important factors driving the choice between centralized and decentralized models at scale.
Decision makers must assess conditions where and when each model (Centralized vs Decentralized) will best support access for a specific CGT product. This article reviews critical factors to consider when making this choice. We conclude that while the decentralized approach may have a place in the manufacturing of CGTs, centralized manufacturing will remain the dominant approach.
Comparative Analysis of the Models
Adopting POC manufacturing requires a rethinking of traditional supply chain dynamics and operational workflows. This shift necessitates the development of compact, automated manufacturing technologies capable of delivering consistent quality across multiple sites. It also requires robust training programs for local technicians and healthcare professionals to manage these advanced systems safely, effectively, and consistently. If applied universally, the POC approach would require the technologies, the training and the staff to be deployed and maintained across a vast network of diverse treatment sites. Regulatory alignment also becomes more complex. The agencies would need to adapt frameworks to accommodate treatment variability across the decentralized production hubs.
Therefore, when choosing between POC and centralized manufacturing for cell and gene therapies, multiple factors need to be considered. The subsequent section outlines the factors that should drive this decision.
Category 1. Manufacturing Costs & Manufacturing Technologies: Cell and gene companies leverage years of laboratory scale process development through pre-clinical studies and then pivotal clinical trials to ultimately reach FDA approval and commercialization. Lowering manufacturing costs is critical for CGT business viability.
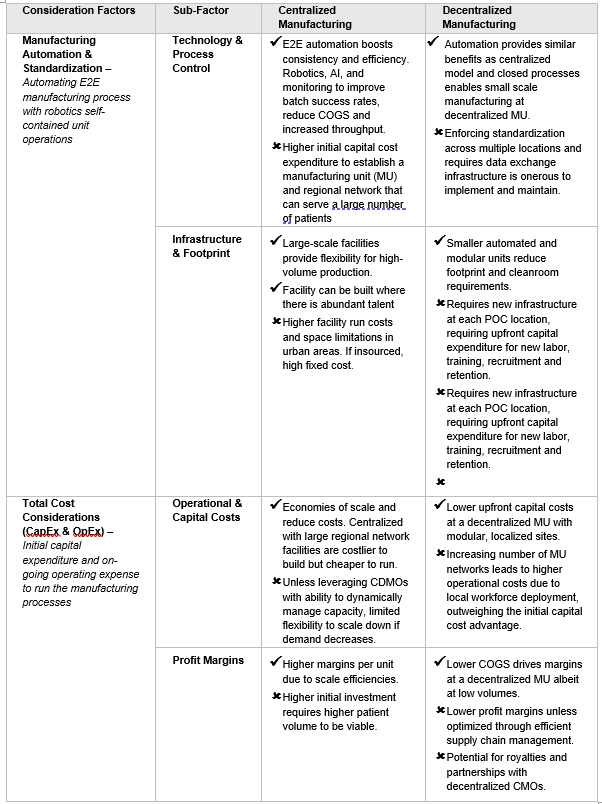
Table 1. Manufacturing Technologies and Economics
Increasing manufacturing efficiency is essential to reducing COGS in CGTs. Automation is a key strategy that can address costs via technology, process standardization, and limiting the size of a facility. Most companies, including new entrants, are adopting automation either directly or with partners. While it raises initial capital expenses, automation significantly reduces labor costs, which constitute 40-50% of COGS3. Closed processes with automation decrease facility footprint and environmental requirements, which enable the production of multiple batches simultaneously. (See Table 1). However, in the POC model, as the number of decentralized units and locations increase, the cost and margin advantage over the centralized model diminish due to operational overhead for maintaining consistent processes across the entire network. The claimed cold chain costs savings in the POC model are overtaken by the dramatically increasing operational overhead costs of an expanding network of mini-manufacturing sites.
Category 2. Operations: The centralized model benefits from an established supply chain, while the decentralized model eliminates the need for shipping patient materials to central facilities (See Table 2).
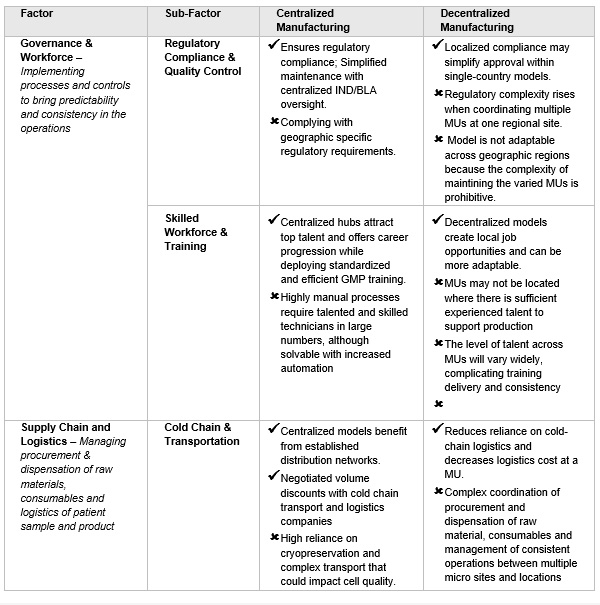
Table 2. Operating Model considerations
Localized manufacturing could enhance equity in patient access, especially when cold chain networks are absent. The decentralized model requires collaboration among hospitals, physicians, and GMP-qualified technicians, overseen by a regulatory agency. This is because multiple site manufacturing requires harmonization of product testing/release and manufacturing. Decentralized manufacturing will be challenging because all centers must use the same manufacturing protocol and, the same or comparable in-process and lot release assays. The quality programs across all centers must be fully aligned. Once commercial, the compliant product validation program will require process performance monitoring and comparison across the network. Outlier sites will need to be addressed in the product annual report. Recently, United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) released a guidance that has paved a way for clinical trial applications to include manufacturing of therapies at point of care. Similarly, under the supervision of the Spanish Agency for Medicines and Health Products (AEMPS), two of Spain's academic hospitals have successfully met these manufacturing requirements. Although other BLA holders could adopt this model, expanding it internationally to form a large network is challenging without harmonized operations and regulations across sites that cross country-specific regulations. Without this, the viability of consistent global commercial or clinical operations for decentralized manufacturing is not plausible.
POC manufacturing units need skilled workers to produce high-quality therapies with automated systems. Each unit will have fewer operators with technical expertise, but overall staffing is proportional to the number of units. Recruiting specialized staff may be difficult, and the staff must be deployed at every POC and fully utilized. The POC model cost savings related to a seemingly smaller workforce compared to the centralized model is more than offset by the need for governance and maintenance of all the staff and equipment that is required to be deployed to each manufacturing unit (MU) node.
Category 3. Patient and Disease Considerations: Centralized and decentralized manufacturing models offer unique advantages to patients and caregivers when aligned with science and technology (See Table 3).
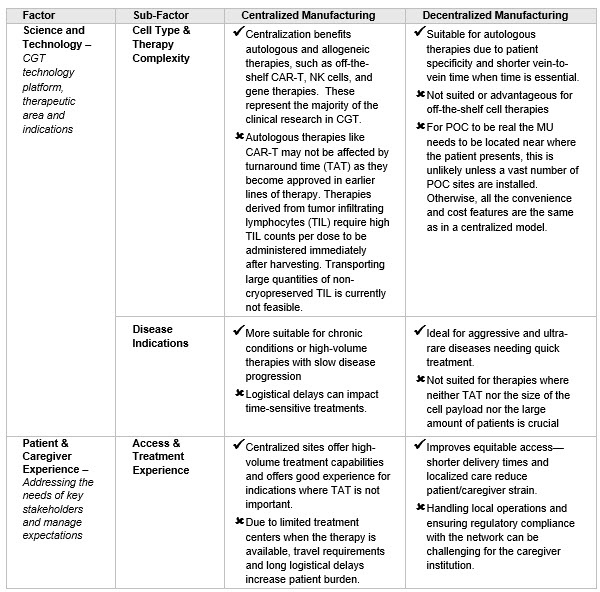
Table 3. Science and Technology impact on customer experience
Choosing between centralized and decentralized manufacturing for therapies depends on dose personalization, technology platform, and patient experience. Centralized models work well for off-the-shelf therapies and less aggressive diseases that don’t require quick treatment. For example, as CAR-Ts move up in the treatment process, turnaround time (TAT) cycle time becomes less crucial. In contrast, decentralized models are better for aggressive diseases or high cell payloads.
In Conclusion
CGT manufacturing is complex despite automated unit operations and GMP reagents. Because of manual interventions to manage disconnected unit operations, companies struggle with scaling up their E2E manufacturing operations. Small-scale automated systems with real-time data analysis and control can aid decentralized manufacturing, but the key is interconnected and harmonized environments across decentralized manufacturing units. Seamless connectivity requires technical expertise and changes in business practices, which will increase overhead. Regulatory harmonization across regions is crucial for implementing common manufacturing practices. Thus, collaboration among technology suppliers, manufacturers, and regulators is essential for decentralized CGT manufacturing success. Without these the model cannot succeed.
Industry leaders, BLA holders, and physicians should assess conditions and asset needs before selecting a point of care or near POC decentralized model. Figure 1 outlines when POC/Near POC models may be preferable to centralized or regional ones.
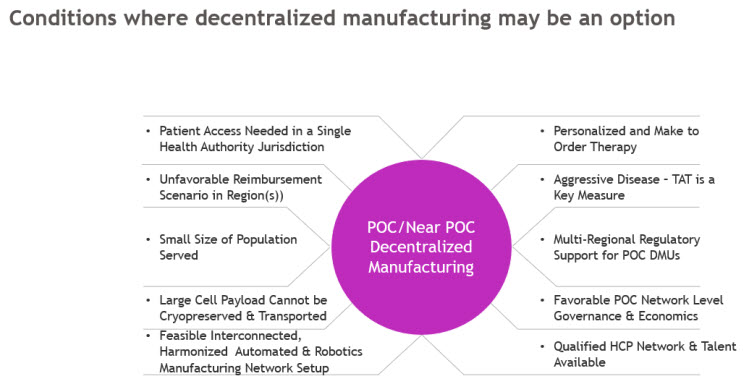
Figure 1. Conditions that must be true to support the viability of the Decentralized Manufacturing Model to be viable
Drug Product Owners must consider multiple simultaneous factors when choosing manufacturing strategies.
- Modality Fit: Therapies requiring fast turnaround or high personalization (e.g., TILs, ultra-rare CAR-Ts) may benefit from decentralized manufacturing models.
- Economic Viability: The Centralized model still offers the best cost-efficiency; decentralization demands high reimbursement or ultra-targeted use.
- Regulatory Feasibility: Regionally harmonized, risk-based regulatory frameworks will be needed before decentralized manufacturing can scale.
- Technology Readiness: Success in the POC model depends on automation, closed systems, and interoperable & interconnected platforms that follow consistent processes across the network.
- Patient Access: Impact of treatment center network design on the size and type of the patient population served.
As illustrated in Figure 1, there are few scenarios where decentralized manufacturing might be applicable. Although feasible, it is unlikely to become the dominant model for commercially available therapies soon; many conditions must be true before a decentralized model can trump the current centralized or regional manufacturing models.
References
- Approved Cellular and Gene Therapy Products, FDA Website, March 2005
- Bringing the curative potential of CAR T-cell therapy to more eligible patients, STATNews, January 10, 2025
- Cost Analysis of Cell Therapy Manufacture: Autologous Cell Therapies, Part 1, BioProcess International, Adriana G. Lopez, et.al. 2018.
Several industry experts contributed to the writing of this article. The authors would like to acknowledge Jason Martin, Senior Vice President and Head of Technical Operations at Elktrofi, for his valuable inputs in shaping this document.
