Shining Light on the Commercial Potential of Optogenetic Therapies
By Kevin Norman, Ph.D., and Peter Bak, Ph.D., Back Bay Life Science Advisors

Fifteen years ago, scientists discovered a method of using light to stimulate light-sensitive receptors in order to turn brain cells “on” and “off” at the flip of a switch. Since that moment, scientists have been using this technique, called optogenetics, as a method of modulating distinct brain circuits with millisecond precision to explore their functional role in numerous complex behaviors, from learning and memory to addiction. Until recently, the use of optogenetics has been completely restricted to use in preclinical animal models; however, advances in the development of optogenetic therapies have allowed this technology to move from the laboratory to humans. The first-in-human use of optogenetic therapies to treat retinal disorders will likely open the gate for utilizing light-activated optogenetic switches to advance the safety and efficacy of treatments for other disorders beyond the eye, including cancer.
Recent Advances In Optogenetics
Optogenetic therapies are currently focused on the treatment of ophthalmology diseases, specifically inherited retinal dystrophies such as retinitis pigmentosa (RP). RP is a class of inherited retinal diseases that leads to the gradual loss of vision in more than 2 million individuals worldwide. RP can be caused by one of 71 different genes that cause the degeneration of retinal rod and cone photoreceptors that normally function to facilitate vision by converting the light that enters the eye into electrical signals that can be decoded by the vision-processing areas of the brain. Besides the 2017 FDA approval of Spark Therapeutics’ gene replacement therapy Luxterna for early-onset RP caused by mutation of the RPE65 gene, there is no approved therapy for RP. While the development of Luxturna has been a major step forward for the treatment of RP, a significant drawback of the therapy is that it is only effective for RP patients with the recessive RPE65 mutation. This only represents approximately 2.5% of the estimated 90,000 individuals in the U.S. suffering from RP, leaving a massive unmet need for the vast majority of patients with RP. Optogenetic therapies have gained traction as a possible “one and done” method for treating multiple forms of inherited retinal dystrophies regardless of the underlying mutation, rather than individually correcting one of the dozens of possible genes that cause loss of retinal photoreceptor processing. Therefore, any patients with blindness caused by the loss of retinal photoreceptors would be candidates to receive administration of an optogenetic therapy within the eye to introduce artificial photoreceptors and restore the eye’s ability to respond to light and relay visual signals to the brain to enable vision.
Recently, GenSight Biologic published findings in Nature Medicine detailing the first demonstration of the clinical efficacy of optogenetic therapies in the ophthalmology space by using its optogenetics platform to partially restore vision in a blind patient with late-stage RP.1 The dual therapy includes an intravitreal injection of a light-sensitive opsin, ChrimsonR, to express the photoreceptor in the retinal ganglion cells in addition to requiring patients to wear light-stimulating goggles that convert ambient light from the patient’s surrounding into a series of specific light wavelengths and intensities that can be projected to the treated eye in real time. After a period of months post-treatment, visual function improved as the lone patient tested was able to perceive, locate, count, and touch objects upon stimulation of the treated eye with the light-stimulation goggles.
The Optogenetics Commercial Landscape
GenSight Biologic is not alone in the race to be the first company to develop an FDA-approved optogenetic therapy for RP (Figure 1). Nanoscope Therapeutics is undergoing Phase 2 clinical trial testing of its novel gene therapy that targets bipolar cells, as compared to retinal ganglion. It aims to convert bipolar cells, which normally alter and relay information from the light-capturing rods and cons to the retinal ganglion, into the “new” photoreceptors of the retina. Nanoscope Therapeutics’ therapy also mechanistically differentiates from GenSight Biologic’s therapy in that the bipolar cells’ photoreceptors are activated by ambient light and don’t require the use of light-stimulating goggles. Preliminary results presented at the 2020 American Academy of Ophthalmology meeting showed that seven of eight patients in the high-dose cohort demonstrated a visual acuity improvement and improved performance in a visually guided mobility test. Another notable emerging optogenetics gene therapy is Bionic Sight’s collaboration with Applied Genetic Therapies Corp. (AGTC). In a Phase 1/2 study of their optogenetic gene therapy, the sponsors claim that the first four blind patients have been able to detect light and motion. Moving forward, the long-term viability of the adoption of optogenetic therapies to treat blindness from either of the aforementioned companies will depend on the quality of vision that can be restored and whether its positive impact on the patient’s quality of life is sufficient to warrant the anticipated high cost of treatment that accompanies gene therapies.
Figure 1. Active Optogenetic Therapy Trials
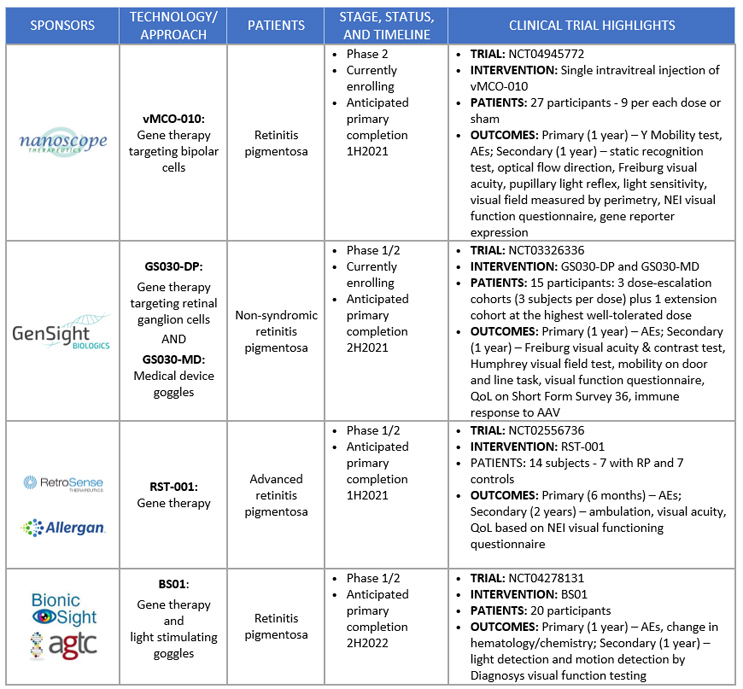
Figures courtesy of Back Bay Life Science Advisors
Biopharma companies have been quick to incorporate optogenetic therapies into their ophthalmology portfolios over the last five years. The first major acquisition in the optogenetic therapy space was in 2016 when Allergan, now an AbbVie company, acquired RetroSense Therapeutics for $60 million in up-front payments with an additional undisclosed amount if clinical and regulatory milestones were reached. Novartis has recently established a strong position in the development of optogenetic therapies through a series of investments. Novartis followed up its October 2020 acquisition of gene therapy startup Vedere Bio ($150 million up front and up to $280 million for reaching clinical milestones) by acquiring Arctos Medical in September 2021 to bolster its optogenetics-based gene therapy program and further expand its presence in the optogenetics space.
Compelling results from translational research have caught the eye of VCs, who have invested nearly $100 million in U.S. optogenetics companies over the last five years, with average Series A funding of $9.3 million, Series A1 funding of $19.6 million, and the lone Series B raising of $30 million in 2021 to MapLight Therapeutics (Figure 2). VC money is being deployed to use optogenetics as both a tool to elucidate brain circuits underlying psychiatric disorders and as a therapy itself.
Figure 2. U.S. Venture Capital Funding in Optogenetics, 2016-2021
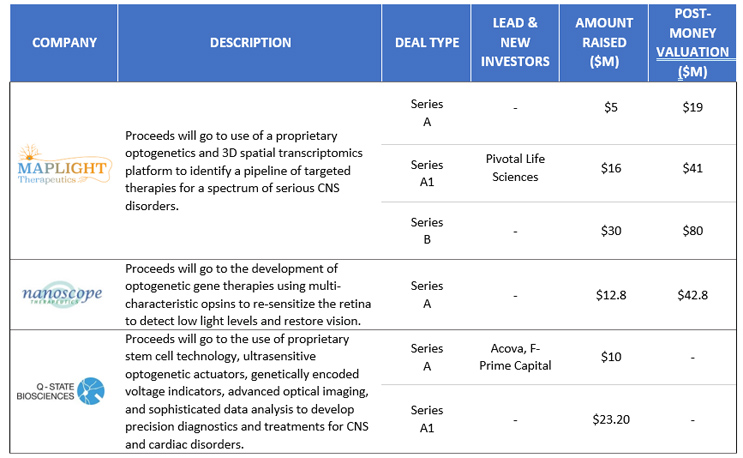
Figures courtesy of Back Bay Life Science Advisors
Future Of Optogenetics
Optogenetics was first developed as a preclinical neuroscience tool and has grown over the last 15 years into a promising approach for the treatment of ophthalmological disorders, but the full therapeutic and commercial potential of optogenetics will likely be unlocked when the technology expands into other clinical domains beyond eye disorders. In addition to chronic pain and cardiovascular diseases, cancer is a particularly intriguing indication where optogenetics could be integrated with current treatments to improve precision and provide a safer and more effective therapeutic.
Five years ago, scientists at Tufts University were the first group to voyage into the field of opto-oncology as they applied optogenetics to both prevent and reverse the progression of cancerous tumors in tadpoles.2 After introducing a photoreceptor to the cell membrane of cells co-expressing an oncogene, they directed light onto the cell to alter the bioelectrical environment of these cells and override the action of the oncogenic mutation to reduce the subsequent formation of tumors. They also demonstrated that optogenetic modulation of pre-formed tumors disrupted the electrical signals that drive cellular communication and reverted dividing cancer cells back to their original non-dividing state. This work has laid the groundwork for potential future uses of optogenetics to modify the bioelectrical components of a dividing tumor cell to control cancer.
More recently, optogenetics has been proposed as a solution to address a key problem limiting the use of CAR T cell immunotherapy in cancer treatment as detailed in Nature Nanotechnology.3 CAR-T therapy involves the engineering of synthetic receptors that function to redirect lymphocytes to identify and destroy cancer cells expressing an individual’s tumor-specific target antigen. The first FDA-approved CAR-T therapy, Kymriah from Novartis, was approved for lymphoblastic leukemia in 2017 and later expanded to B cell lymphoma. Despite successes as a treatment for cancer, the therapeutic efficacy of CAR-T therapies in solid tumors and hematological malignancies is limited by safety concerns from multiple factors, including lack of spatiotemporal control of anti-CD19 CAR-T cell activity that can lead to cytokine release syndrome (CRS) and damage of healthy B-cell lymphocytes, called B cell aplasia.
In order to address the limited spatiotemporal control of CAR-T therapy, researchers at Texas A&M University turned to optogenetics and engineered photosensitive CAR-T cells that only function in the presence of particular light wavelengths and the specific tumor antigen. To this end, they integrated optogenetic receptors with a similar anti-CD19 CAR-T construct as Kymriah, creating CAR-T cells that are only activated when triggered by deep tissue-penetrating near-infrared light. In a series of experiments, mice were injected with cancerous cells and either localized or systemic administration of photoinducible CAR-T cells before receiving eight to nine days of tumor-localized light stimulation for 2 hours (cycles of 20 seconds on and 5 minutes off) each day. Using this novel optogenetic CAR-T technology, researchers were able to limit the growth of solid tumor melanoma and lymphoma cells while mitigating safety side effects associated with non-specific CAR-T, specifically the elevation of interleukin-6 (IL-6) a mediator of CRS.
The development of optogenetic therapies for use in oncology or other indications, such as chronic pain or cardiovascular disease, may face multiple clinical and commercial hurdles. While the expression of optogenetic constructs in the retina relied on localized non-specific expression in retinal ganglion cells, the use of optogenetics for pain management would likely require a more selective expression in a subpopulation of cells to reduce off-target side effects. An optogenetics based approach could differentiate from conventional spine electrical stimulation by targeting specific cell types, such as unique classes of afferent sensory projections originating from the sources of pain. However, this would necessitate a degree of specificity beyond choosing an appropriate AAV serotype or cell-type specific promoter and may be better suitable for a Cre-Lox control system available only in preclinical models and would come with the significant trade-off of lower opsin expression. For any application of optogenetics, an efficient, adjustable, and durable light delivery system is crucial and future advances will need to optimize this component. From a commercial perspective, the uptake of this gene therapy-medical device combination will be limited by healthcare providers heavily managing the use of the therapy as a result of its anticipated high cost. Even for electrical stimulators, healthcare providers require physicians to temporarily implant the device and provide documentation of at least 50% reduction in the patient’s pain before authorizing the device to be permanently implanted.4 To date, cardiac and pain management optogenetics remain in a nascent preclinical stage and overcoming these obstacles will be a key step in providing an opportunity for clinical translation.
About The Authors:
 Kevin Norman, Ph.D., is a consultant at Back Bay Life Science Advisors, where he is part of an integrated strategy and investment banking team supporting biopharma, medtech, and healthtech development. He has conducted clinical and preclinical research throughout his research career at Tufts University, McLean Hospital of Harvard Medical School, and the Icahn School of Medicine at Mount Sinai. Norman has published more than 10 peer-reviewed scientific articles using optogenetic, pharmacological, and behavioral approaches to investigate a range of topics. He has expertise in a multiple neuro/CNS indications, including autism, drug addiction, and depression.
Kevin Norman, Ph.D., is a consultant at Back Bay Life Science Advisors, where he is part of an integrated strategy and investment banking team supporting biopharma, medtech, and healthtech development. He has conducted clinical and preclinical research throughout his research career at Tufts University, McLean Hospital of Harvard Medical School, and the Icahn School of Medicine at Mount Sinai. Norman has published more than 10 peer-reviewed scientific articles using optogenetic, pharmacological, and behavioral approaches to investigate a range of topics. He has expertise in a multiple neuro/CNS indications, including autism, drug addiction, and depression.
 Peter Bak, Ph.D., is managing director at Back Bay Life Science Advisors. He has more than 10 years of experience with a broad range of research approaches—cellular, molecular, and biochemical—and in fields from immunology and infection through oncology. He leads projects for global life science developers, focusing on liquidity planning and positioning, strategic franchise building, M&A and licensing strategy, and buy-side diligence. Bak’s research career in immuno-oncology began at Dartmouth Medical School, and his research continued at MIT as an American Cancer Society Postdoctoral Fellow at the Koch Institute of Integrative Cancer Research. He has published peer-reviewed articles on cancer vaccinology and other topics.
Peter Bak, Ph.D., is managing director at Back Bay Life Science Advisors. He has more than 10 years of experience with a broad range of research approaches—cellular, molecular, and biochemical—and in fields from immunology and infection through oncology. He leads projects for global life science developers, focusing on liquidity planning and positioning, strategic franchise building, M&A and licensing strategy, and buy-side diligence. Bak’s research career in immuno-oncology began at Dartmouth Medical School, and his research continued at MIT as an American Cancer Society Postdoctoral Fellow at the Koch Institute of Integrative Cancer Research. He has published peer-reviewed articles on cancer vaccinology and other topics.
Connect with the authors at www.bblsa.com.
- Nature Medicine. 27, pages 1223–1229 (2021) (https://www.nature.com/articles/s41591-021-01351-4)
- Oncotarget. 2016 Apr 12; 7(15): 19575–19588 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4991402/)
- Nature Nanotechnology. (2021). Online ahead of print (https://www.nature.com/articles/s41565-021-00982-5)
- United Healthcare Plan Implantable Electrical Stimulator for Spinal Cord Prior Authorization form
