RNA Cell Therapy: A New Class of Advanced Therapies
By Murat Kalayoglu, MD, PhD, President & Chief Executive Officer at Cartesian Therapeutics

RNA cell therapy is a new class of advanced therapies now in clinical trials to treat an array of diseases in and beyond oncology. These therapies combine the best features of two therapeutics modalities, conventional RNA therapy and cell therapy, while avoiding key drawbacks. With RNA cell therapy, there is no need to package RNA into lipid nanoparticles, and there is precise control over which cell type receives the RNA. Likewise, there is no need to alter cells permanently with DNA, as is done with conventional cell therapy. The result is a potent yet safer cell therapy that enables combination therapy, delivery of that combination directly to the site of disease with targeting proteins encoded within the cell, and sustained release of therapeutic proteins by co-opting the cell to serve as an in vivo “drug factory.”
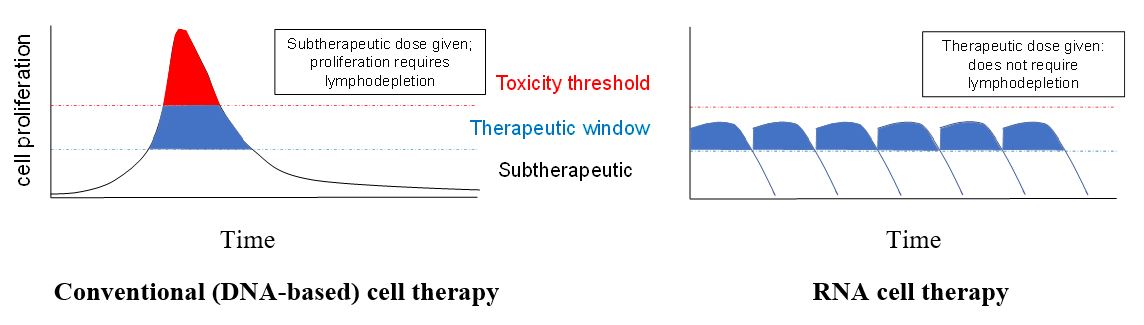
RNA cell therapies were developed to address the shortcomings of conventional (DNA-based) cell therapies, in particular their toxicity. The classic example of a conventionally engineered cell therapy is chimeric antigen receptor (CAR) T-cells, which are engineered with DNA encoding a chimeric protein consisting of an external antigen-binding domain linked to an internal cell-activating domain. Because the CAR is irreversibly encoded in the T-cell’s DNA, every CAR T-cell division yields daughter cells genetically identical to the parent cell. As such, conventional, DNA-modified cell therapies have unpredictable pharmacokinetics. CAR T-cells are engineered ex vivo, and conventionally, they are administered to patients at subtherapeutic doses, with the expectation that the cells will proliferate up to a therapeutic window and remain there for an extended period (Figure 1). But there is no inherent check on the proliferation of these conventional CAR T-cells, which can continue to proliferate to toxic levels. Therefore, conventional, DNA-modified CAR T-cell therapies carry an inextricable risk of toxicities such as cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and infections. DNA-modified CAR T-cells also carry long-term risks including transformation, immunogenicity, and delayed infections. Patients receiving such therapies must be monitored for at least 10 years to assess these long-term risks. In addition, the in vivo proliferation required by DNA-modified CAR T-cells only occurs if patients first receive preconditioning (lymphodepleting) chemotherapy. But this lymphodepleting chemotherapy is toxic in and of itself, leading to sustained immunosuppression over weeks. These risks might be acceptable when treating relapsed/refractory cancer; however, a less toxic approach is needed if cell therapies are to be administered as frontline therapy (i.e., to newly diagnosed cancer patients) or beyond oncology.
The RNA Safety Profile
To overcome the limitations of DNA-modified cells, RNA cell therapy introduces one or more isolated, well-characterized RNA templates into purified cells. Because RNA degrades naturally over time, RNA cell therapies are time-limited and possess drug-like pharmacokinetics. Cells therefore can be dosed repetitively, at the therapeutic window (Figure 1), and each cell division leads to a theoretical halving of the functional RNA and RNA-encoded proteins within the cell. As such, if the cell begins to proliferate beyond the therapeutic window, it quickly becomes less functional, ensuring that a “break” is applied to the therapy. This is a key feature of RNA cell therapies that translates to their enhanced safety profile. In addition, given that cells are administered directly at the therapeutic window and not at subtherapeutic doses with an expectation that they will eventually proliferate into the therapeutic window, RNA cell therapies do not require the toxic lymphodepleting chemotherapy that conventional (DNA-based) cell therapies do. Also, because RNA does not integrate into the genome, it poses no risk of malignant transformation. The enhanced safety profile of RNA cell therapies allows them to be used beyond advanced cancers, for example, in frontline oncology as well as beyond oncology in autoimmune, respiratory, and cardiovascular disorders. In addition, compared to conventional (DNA-based) cell therapies, RNA cell therapies are significantly less expensive to manufacture. Their lower risk of toxicity translates to lower costs of monitoring for, and treating, short- and long-term toxicities.
RNA cell therapies are currently being tested in multiple clinical trials. Cartesian Therapeutics, a pioneer in RNA cell therapy, has six assets in its pipeline, each developed on the company’s proprietary RNA Armory cell therapy platform. The overarching vision behind the RNA Armory® is to RNA-engineer any cell, to target to any tissue, and to express a combination of therapeutic proteins or targeting molecules. In this regard, the cell serves both as a factory for producing, and a vehicle for delivering, multiple therapeutic proteins directly to the site of disease. These therapeutic proteins can be newly engineered proteins, physiologic (wildtype) molecules, or a combination of both. As such, the disease pathway can be treated at multiple nodes simultaneously with a combination of therapies. Three of Cartesian’s assets are currently in clinical trials treating patients with oncologic, autoimmune, and respiratory disorders. Descartes-08 is the first RNA cell therapy in an autoimmune disease, treating patients with generalized myasthenia gravis. Descartes-11 is the first RNA cell therapy in a frontline cancer, treating patients with high-risk, newly diagnosed multiple myeloma. Descartes-30 is the first RNA cell therapy in a respiratory disease, treating patients with acute respiratory distress syndrome (ARDS), including patients with COVID-19, influenza-related, and non-infectious ARDS. Cartesian’s preclinical assets are third-generation products engineered to express three or more therapeutic and targeting proteins. Other groups are also engineering cells with RNA. Myeloid Therapeutics is targeting advanced cancers, such as glioblastoma multiforme, with RNA-engineered macrophages. Maxcyte is using RNA-engineered CAR T-cells to target advanced ovarian cancer. Multiple academic groups are also developing RNA cell therapies to treat a variety of cancers.
Just as mRNA vaccines have provided a safe, rapid, versatile, and economical technology for future vaccine development, there are reasons to believe that RNA cell therapy will provide similar benefits. Clinical trials already underway will inform us on which diseases benefit most from RNA cell therapy. Perhaps most exciting, the relative safety of RNA cell therapies is allowing biotech to advance precise, potent cell therapies beyond oncology.
Figure 1. Relationship of cell proliferation to toxicity for conventional (DNA-based) cell therapy versus RNA cell therapy. See text for details.