Rethinking Cell Therapy Durability: Transient, Non-genetic, Changes To Cell Function Could Be Key To Broader Patient Impact
By Armon Sharei, Ph.D., CEO, SQZ Biotechnologies
The preferred outcome of any therapeutic is to provide durable clinical benefit without the need for chronic treatment. Cell and gene therapies are no exception. In fact, much of the appeal of certain cell and gene therapy strategies is that they could potentially provide multi-year or lifelong patient benefit from a single course of treatment. The field has accomplished much with current strategies; however, durable patient benefit need not be synonymous with durable cell engineering.
While the preference for durable outcomes is indisputable, the biological path to generating that outcome need not necessarily involve permanent changes to a cell. Approved gene therapies for certain diseases, such as ALD and SCID, entail permanent modification of patient hematopoietic cells with a viral vector encoding the therapeutic transgene. While they have demonstrated therapeutic benefit, these therapeutics also carry serious safety risks due to the potential for undesirable genetic changes that can lead to oncogenesis or other complications. In contrast, childhood vaccines, such as the hepatitis B vaccine, provide life-long protection against the virus by only transiently exposing the immune system to the target antigen and an adjuvant for a relatively brief period. The resulting immunological memory provides durable protection without the need for the therapeutic to directly induce a genetic alteration. In fact, our physiological mechanisms to respond and overcome a range of ailments—be it infections, wounds, etc.—almost never involve a genetic change. Thus, as we consider the future of cell and gene therapies, strategies that leverage transient cell alterations to enable a durable benefit can be a major driver of patient impact.
There are a few key limitations to permanent (usually genetic) cell alterations that limit the field’s impact:
- Long-term Safety Concerns: Any strategy that relies on changing the genome of a patient’s cells ultimately begs the question, did it break something? Even low probability genetic events carry a meaningful risk of promoting future malignancies or other complications. This concern can limit both the indications for which cell and gene therapies can be used and the line of treatment for which they would likely be adopted. Few patients or physicians would tolerate this long-term risk for non-life-threatening diseases or even serious disease settings with established alternatives.
- Durable Side-Effects: If the genetic alteration outlasts the disease, it may continue to drive complications long after the disease is gone. For example, there was recent exciting news about a CAR-T patient that had detectable CARs and remained disease free after ten years. However, this patient also remained B cell-free. In other words, they are immunocompromised for life because their durable CAR-T response is continuing to kill their B cells—malignant or not. In contrast, the typical first line treatment for B cell malignancies (such as the chemotherapy combination, R-CHOP) only temporarily ablates B cells, often curing the patient, and eventually allowing the normal population to re-establish function while limiting any serious long-term side-effects.
- Limited Biology and Disease Applicability: While there are some therapeutic strategies that are amenable to permanent expression of a gene, there can be many situations where transient expression is preferable or required. For example, CAR-Ts have struggled with toxicity issues in patients with non-B cell malignancies often because the desired target (e.g., HER2) is expressed on critical non-malignant cells. A therapeutic strategy that actively targets disease based on HER2 for a limited time could provide a desirable therapeutic window while those that continuously attack those cells have had sometimes fatal consequences. Similarly, some powerful immune modulators like IL-12 have been expressed in TIL therapies – such a potent cytokine is attractive for transiently boosting activity, but its permanent expression proved to be fatally over-stimulatory. A transient expression strategy can potentially allow one to create more tunable therapeutics with broader potential for impact.
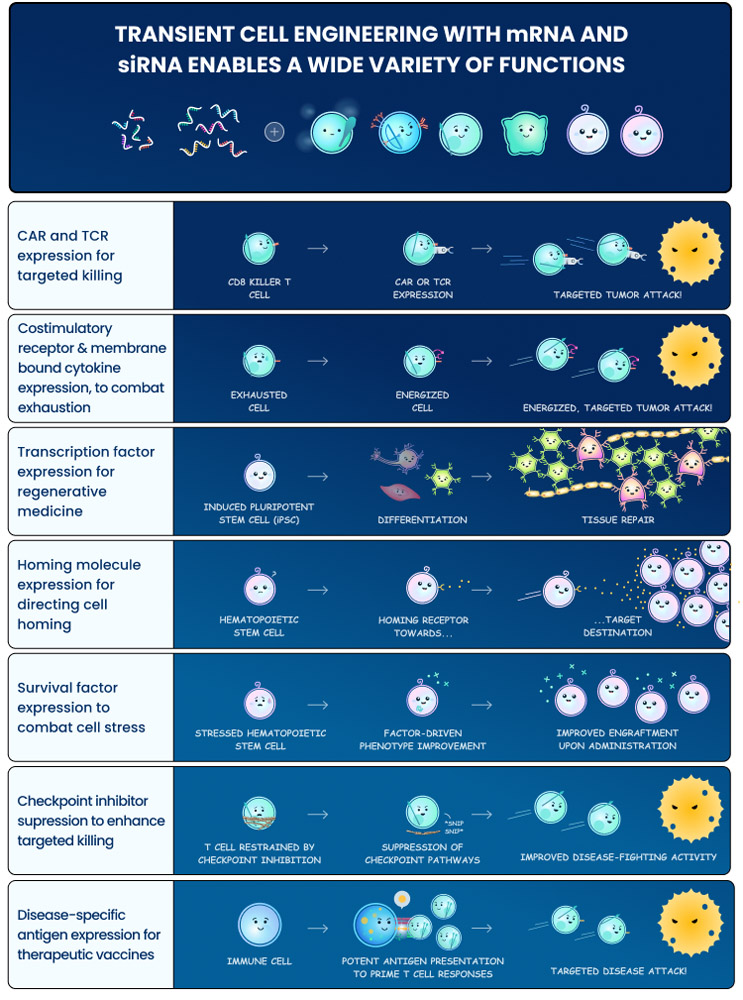
With advances in cell engineering technologies and transient expression cargos, the field now has the opportunity to create cell therapies that have broad potential beyond the initial generation. The Cell Squeeze® technology, for example, enables robust delivery of virtually any therapeutic cargo, or combinations of them, into many cell types in a rapid, cost-effective manner. Molecular tools for temporary modulation, such as mRNA and siRNA, have also become increasingly more robust and practical to manufacture. The marriage of these types of technologies potentially could allow one to express or suppress pathways of interest to create highly sophisticated cell therapies that impact the body through a variety of mechanisms and can last days or hours before reverting back to their native state. The rapid manufacturing, desirable safety attributes, and potential point-of-care implementation of technologies like Cell Squeeze® suggests that such therapies could be broadly accessible beyond major academic treatment centers.
There are many exciting therapeutic strategies one can envision by adopting a transient manipulation strategy:
- Transient CAR/TCR Therapies: One could use mRNA expression to create CAR or TCR therapies that are active for a few days before repeat dosing for additional cycles. This type of strategy could enable several potential advantages relative to the existing permanent expression approaches:
- In current applications, such as hematological malignancies, it could enable tuning of dose levels across treatment cycles to mitigate side effects - because an mRNA-based receptor will inevitably fade. Currently, because of the ‘one and done’ approach, there is no true opportunity to modify treatment potency. Moreover, viruses can lead to high expression variability across batches due to the complex dynamics of viral gene integration. Delivery of mRNA, by contrast, could potentially be more consistent.
- Transient expression can mean that the therapeutic activity stops when the dosing stops. If a patient has cleared the malignancy, one could potentially stop administration and allow the endogenous B cell or other target population to rebound. This is similar to current early-line biologics strategies targeting CD20, HER2, BCMA, among other mutations, that don’t permanently destroy their target populations.
- One could leverage powerful immune activating biologies, such as membrane-bound cytokines, or survival enhancers to increase CAR, TCR, or TIL function and drive more potent activity for a short period of time. Many of these attractive enhancements would be too dangerous to deploy in a permanent expression format.
- Vaccination and Tolerization Modalities: Antigen presentation mechanisms capable of activating or suppressing an endogenous immune response against a target antigen have exciting therapeutic potential but have been difficult to implement to date. In fact, the immune system is designed to react most productively to transient antigen expression. For example, traditional vaccine modalities are very effective in numerous prophylactic settings and achieve this through temporary exposure of the system to the target antigen. If one continuously expressed a hepB or COVID antigen, for example, it would prompt over-activation and eventual exhaustion of the immune response instead of the durable memory-based protection that is desired. By enabling transient engineering of antigen presentation mechanisms, such as approaches taken with the SQZ® APCs, AACs, and eAPCs, one can potentially leverage the power of immunological memory to combat a broad range of oncologic and infectious disease indications. These approaches use simultaneous delivery of multiple payloads to provide robust MHC-I presentation of target antigen while upregulating costimulatory signals from surface receptors and cytokines to coincide temporally with antigen presentation and thus drive powerful T cell responses. The mirror of these applications is for immune tolerance in autoimmune diseases. The SQZ® TACs, for example, leverage engineered RBCs to drive transient expression of antigens in a tolerogenic context and induce potentially durable tolerization of an autoimmune response.
- Cell Reprogramming: The field of regenerative medicine ultimately relies on the direction of cell fate to achieve therapeutic impact. One must be able to induce the creation of specialized neurons, muscle tissue, cartilage, stem cells, etc. However, a critical challenge is that the transcription factors that determine these cell fates must be expressed at a well-regulated level, and many may only be desirable for short periods of time. Often, a permanent expression of such fate-determining transcription factors can result in undesirable cell behavior/function. Our bodies have generally evolved to use subtle mixtures of signals to modulate these transcription factors during development without relying on any genetic changes. Through the use of transient transcription factor expression and suppression strategies (e.g., with mRNA and siRNA), one could potentially engineer cell fate and create cellular therapeutics for a broad range of applications. A field that has historically faced serious challenges to driving therapeutic impact may finally realize its full potential.
While the field has accomplished much with current strategies that rely on permanent cell alteration, durable patient benefit is not synonymous with durable cell engineering. Many therapeutic approaches could potentially be better served with transient expression, and it could lead to broader impact by enabling new therapeutics that were infeasible in the binary world of gene integration and gene deletion. Advances in manufacturing that could significantly improve the accessibility and cost-effectiveness of cell therapies would further enable these newer therapeutics, ultimately allowing for broader patient impact through an expanding multitude of complex biologies only attainable through a cellular therapeutic.
