Rare Diseases' Cost Burden On Patients
By Giacomo Chiesi and Gina Cioffi, Chiesi Global Rare Diseases

There are an estimated 400,000 people globally affected by more than 7,000 known rare diseases, most of them with genetic origins.1 In recent years, many companies have worked to address the underlying causes of a range of rare diseases by developing innovative therapies with the promise of fulfilling significant unmet needs of patients and families and bringing them safe and effective treatments. Several innovative therapies have achieved FDA and market approval, but still 95% of rare diseases do not have approved treatments or experimental therapies under investigation.
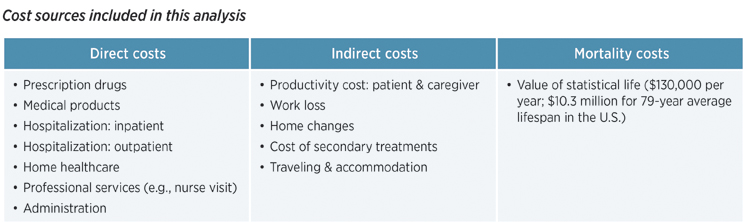
Patients, families, and caregivers often bear the brunt of costs directly and indirectly, whether it be medical costs for supportive or palliative care, medical procedures and hospitalizations or travel for medical visits, home modifications, and work or productivity loss. By understanding the cost drivers and economic impact that a lack of available treatments poses for rare disease patients, pharmaceutical companies, government leaders, policy makers, society, and other stakeholders can identify the unmet needs in the rare disease community and how those needs may be addressed in the years ahead.
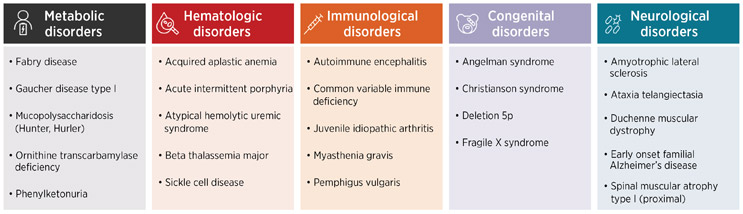
Our team at Chiesi Global Rare Diseases, with support from IQVIA, set out to evaluate the direct, indirect, and mortality-related costs for 24 rare diseases across five therapeutic areas – metabolic, hematologic, immunological, congenital, and neurological – to assess the burden of care when treatment is or is not available. We compared these costs with common mass market diseases such as diabetes, cardiovascular disease, Alzheimer’s disease, different types of cancer, and arthritis.
Overview Of Healthcare Costs
We found that a lack of treatment for a rare disease is associated with a 21.2% increase, on average, in total costs per patient per year (PPPY) based on the 24 rare diseases reviewed in our analysis. The percent change ranged from a 2.2% increase for congenital diseases to a 51.8% increase for metabolic diseases. Overall, the total cost to society for the 24 diseases investigated is approximately $125 billion, with an economic burden PPPY ranging from $121,000 to $334,000 – this is approximately 10x the cost associated with mass market diseases. The economic burden was generally driven by direct and mortality costs and was highest for metabolic ($334,000 PPPY) and neurological disorders ($317,000 PPPY). Among our findings:
- Direct costs were shown to be $63,000 PPPY with treatment vs. $118,000 PPPY without treatment.
- Indirect costs were $40,000 PPPY with treatment vs. $73,000 PPPY without treatment.
- Mortality costs were $36,000 PPPY with treatment vs. $49,000 PPPY without treatment.
Importantly, the availability of treatments generates value by shifting the burden related to indirect costs from patients and caregivers into direct costs, which are more likely to be covered by private and public payers, including Medicare and Medicaid programs.
3 Strategies To Improve Cost Burden
This study provides an important benchmark for cost disparities and how the burden of rare diseases is impacted by treatment availability. While the magnitude of the cost burden of rare diseases is staggering, there are potential solutions and steps that drug developers can take moving forward.
1. Invest In Rare Disease Research And Development, With Diversity In Mind
Companies should invest in the research and development (R&D) of safe and effective therapies for a range of rare diseases and prioritize these efforts. Many companies may hesitate to focus on rare disease drug development, in part, because of a lack of specialized expertise or a fear of not recouping the costs of investment given the small treatment-eligible patient populations. But analyses including ours show that research and support for rare disease patients makes strong economic sense. Industry must also take the necessary steps to understand and address the need for diverse patient cohorts in clinical trials and be prepared to meet societal concerns for health equity and diversity in terms of both R&D and business strategy.
Drug developers may consider pursuing multi-indication platform technologies or combination therapy approaches to develop treatments. There are also incentives in place that encourage innovation, such as the Priority Review Voucher (extended until 2026), the Orphan Drug Tax Credit (restored to 50% and without limited application), faster access to market (via FDA fast track designation and accelerated approval), and longer market exclusivity (via FDA orphan drug designation) for rare disease drugs. As we now have evidence that rare disease treatments, when approved as safe and effective, generate societal value, companies must build awareness around such value and seek public funding.
2. Communicate With Government Leaders And Policy Makers
Drug developers might also invest time and effort in proactively starting discussions with government leaders, including members of Congress and state elected representatives to try to influence changes in healthcare policies that could lead to wider access to diagnostics for infants and children. Fostering steps toward modern newborn screening systems is imperative. The current capacity of newborn screening systems is limited to adding two new diseases to screening panels per year. Our Advisory Committee on Heritable Disorders in Newborns and Children will never be able to respond to the progress being made in cell and gene therapy at this rate, and industry must take a lead in discussions to meet this objective.
3. Lead Future Studies On Economic Burden
Our findings complement prior research on the economic burden of rare diseases and present an economic tool for analysis of the positive impact of rare disease treatments, but there are some study limitations, and this is also just the tip of the iceberg. There remains a need for further studies in this area. Other drug developers might consider conducting future studies, as there are many factors and healthcare costs at play, to gain a full picture of the overall economic burden and be able to develop strategies to better support rare disease patients. Collecting economic data also offers scenarios so that policy makers can fully understand the benefit of investing in innovation and policy reforms to accelerate the availability of, and access to, rare disease treatments.
Conclusion
There is a shared societal responsibility to provide “health for all at all ages” – one of the United Nations’ 17 Sustainable Development Goals (SDGs) – that should not exclude patients with rare diseases. As a family business and a certified benefit corporation, we are invested in and focused on the long-term support of people impacted by rare diseases. It is essential that other drug developers invest in bringing new therapies to market, better understanding areas of unmet need and how to address them, and collaborating with a range of industry stakeholders including government bodies, policy makers, and advocacy organizations to improve treatment access. Industry must also be able to take a long-term approach to research and support for the rare disease community to foster a cooperative dynamic among all stakeholders and compel transformative action on behalf of the patient community.
References
1. Global Genes. RARE Disease Facts. Retrieved March 9, 2022, from https://globalgenes.org/rare-disease-facts/
 About The Authors:
About The Authors:
Giacomo Chiesi is head of Global Rare Diseases at the Chiesi Group, where he leads the team developing and commercializing treatments for rare and ultra-rare diseases.

Gina Cioffi is senior manager of public affairs at Chiesi Global Rare Diseases, where she advocates for people, caregivers, and families impacted by rare diseases and conditions.
