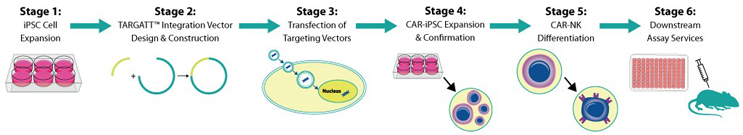
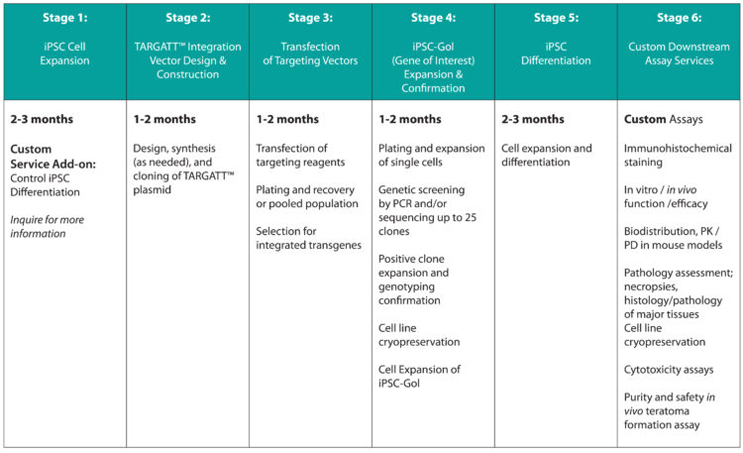
Quality GMP-Grade iPSC And Knockin-Ready TARGATT™ iPSC

To jump start iPSC derived therapeutic cell product development; achieve reliable and efficient genome integration with our innovative solutions.
GMP iPSC
Our high-quality GMP-grade iPSCs (ASE-9280) were reprogrammed from CD34+ male cord blood using an episomal reprogramming method and have proven gene editing and differentiation capabilities. The ready-to-use ASE-9280 GMP line and matching research-grade iPSCs (ASE-9250) are available for purchase. Expedite your project timelines by placing your order today!
- Source cell type: CD34+ Cord Blood, healthy male
- Reprogramming method: Episomal
- Master cell bank or working cell bank available
- Seed cell bank has Drug master file with US FDA
- Matching Research iPSC Line (ASE-9250) is Available
- Proven to be genomic stable (karyotype normal) and transgene expression stable post gene editing and differentiation
- No additional passthrough cost
- Partner with CIRM (California Institute of Regenerative Medicine) in supplying GMP grade iPSC lines
cGMP-Grade TARGATT™ iPSC (Gene Knock-in Ready)
Are you looking for a starting GMP-grade iPSC line that permits safe and efficient insertion of large DNA fragments for your drug development projects?
ASC’s GMP-Grade TARGATT™ Master iPSCs (ASE-9480) are ready for site-specific knock-in of any gene of interest (GOI) up to 20 kb in size.
These enhanced iPSCs were generated from our GMP iPSC Line, ASE-9280, and contain a TARGATT™ landing pad for unidirectional gene insertion catalyzed by the TARGATT™ integrase.
Learn how TARGATT™ works>
After DNA knock-in, TARGATT™ iPSCs can be differentiated into a lineage-committed cell type—neural progenitor cells, astrocytes, dopaminergic neurons, cortical neurons, cardiomyocytes, NK, T cell, hematopoietic stem cells, and more—to enable studies that better replicate the in vivo biology.
cGMP-Grade TARGATT™ iPSC are:
- Engineered from ASC’s GMP grade iPSCs (ASE-9280) using MAD7 (No CRISPR/Cas9)
- GMP SOP
- All reagents are CGT grade (animal, serum-free)
- Documentation and QC/QA follow our eQMS
- Certificate of Analysis provided upon request
- All testing follows GMP guidelines
- License-ready and clear IP path to commercialization
- GMP-Matching research TARGATT™ iPSCs (ASE-9450) are available
Why Applied StemCell?
We understand working with iPSCs remains difficult, and you may encounter several challenges. At ASC our stem cell and gene editing experts are ready to help you every step of the way.
- Send in your construct design for review. Our experts can help you enhance your design.
- Our team can design research and GMP projects in parallel OR generate a matching research grade cell line for preliminary testing.
- Downstream assay services are available: cell-based assays, in vivo animal model testing / PK / PD / ADME, and more.
- Regulatory filing support: CMC / IND support.