Operationalizing Global Oncology Trials

Catalyst Oncology accelerates innovation by aligning strategic insight, deep expertise, and flawless execution. With global reach, we fast-track development timelines through a seamlessly integrated approach, empowering biotechs to deliver life-changing therapies to patients worldwide—faster, smarter, and with purpose.
Critical considerations for global trial success
Executing a trial across regions requires more than operational readiness—it demands strategic planning across regulatory, cultural, and logistical dimensions. The following considerations help ensure precision and speed throughout trial design and execution.
- Smart Trial Designs: Adaptive protocols, PK/PD strategies aligned with Project Optimus and expansion cohorts.
- IP & Sample Logistics: Cold chain management, sample integrity, and contingency planning for global supply.
- Global Execution Strategy: Multiregional planning with regulatory insight, infrastructure readiness, and cultural fluency.
- Risk-Based Monitoring & Safety Oversight: Centralized analytics and proactive reviews to mitigate risk and protect patient safety.
- Study Startup & KOL Engagement: Targeted site selection and early KOL input to drive recruitment and visibility.
- Communication & Vendor Oversight: Transparent leadership and integrated dashboards for team alignment.
- Data Driven Approach: Real-time data to support agile decisions across dynamic study arms.
- Data Privacy & Regulatory Compliance: Governance aligned with GDPR, HIPAA, and global standards.
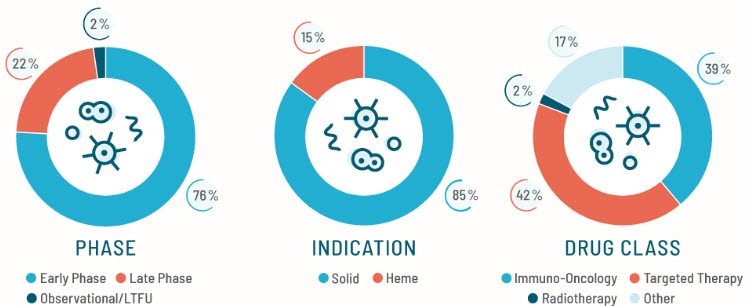
Global Footprint to Support Early- to Late-phase Trials
Catalyst Oncology supports global trials from FIH to registrational Phase III with 1,000+ staff across North America, Europe, and Asia-Pacific. Our global infrastructure enables seamless execution of oncology studies at every stage of development.
Exclusively biotech-focused
Built to work exclusively with biotechs, our flexible processes and approach allow us to listen first, align goals, and execute with an eye toward rapid shifts when a protocol is amended or Breakthrough designation moves a product straight from Phase I to registration.
Seasoned oncology experts
With an average of 9+ years of oncology experience for key roles (PM, CTL, DM, Bios, Clinical Science), our oncology specialty guarantees you a seasoned team across all functions.
A transparent people-first culture with industry-leading retention
We are responsive and attentive to the needs of both our customers and staff. Our industry-leading employee and project team retention guarantees program continuity, increased efficiency, and happy investigative sites.
Active next-gen oncology experience
Working both locally and globally across Phases I through III in a range of solid tumor and hematologic indications, we understand the nuances of complex study designs, novel endpoints, and cutting-edge technologies.