Non-Viral In Vivo Transfection - Cationic Lipids For LNP Formulation

LipidBrick® IM21.7c
- Efficient: Modulates LNP properties to adapt the biodistribution depending on the therapeutic purpose
- Secure: Use a unique lipid structure protected by an independent patent owned by Polyplus®
- Accelerate your project: in vitro and in vivo proof of concept have been successfully performed
Specifications
|
Name |
LipidBrick® IM21.7c |
|---|---|
|
Type |
Cationic lipid |
|
Linear Formula |
C59H117ClN2 |
|
Molecular weight |
890.03 g/mol |
|
CAS |
2416939-42-7 |
|
Storage |
- 20°C |
Summary
DNA and RNA therapeutics are drug products composed of an active genetic component linked to an efficient delivery system, generallyLipid NanoParticles – LNP. Those treatments include both prophylactic vaccines that prevent an infection by triggering the patient’s immune system and therapeutic vaccines that will be used to cure a patient from a specific disease. Each therapy will require its own LNP formulation depending on the genetic material and the targeted tissue. To adapt the delivery system to the need, a wide variety of lipids that modulate the encapsulation efficiency and the LNP properties is needed for therapeutic success.
As an innovator in the delivery field, Polyplus developed a range of cationic lipids used in LNP formulation named LipidBrick®. Those “active lipids” will protect nucleic acids such as DNA, mRNA and siRNA and deliver them to targeted cells. By adding positive charges to an LNP, LipidBrick® broadens the current applications spectrum in terms of potency and targeting, as LNP electric charge is known to impact biodistribution and expression of mRNA-LNPs. LipidBrick® modifies the overall charge of the LNP, allowing to adapt the biodistribution.
Polyplus’ goal is to support customers from R&D to commercialization. LipidBrick® has been patented by Polyplus to avoid any patent infringement that could set back therapy advance.
LNP formulation
Lipid Nano Particles – LNP are the non-viral delivery system of choice for DNA and RNA therapeutics. The lipidic structure enables delivery of nucleic acids into cells using several administration routes (systemic or local injection). LNPs offer a great stability over the time that is essential for a drug product.
LNP are composed of 3 types of molecules:
- Active lipids (cationic and/or ionizable lipids): positively charged lipids will interact with negatively charged nucleic acids
- Structural lipids (phospholipids): Provide LNP rigidity and stability
- Modulators:
- PEG-lipids: Provide steric stabilization and prolong blood circulation
- Cholesterol: Increase membrane fluidity and stability, promote membrane fusion
The manufacturing process of LNP is made using microfluidic systems that will homogenize the nucleic acids (mRNA, DNA, siRNA, or other types) and the lipid mixture in a laminar flow. Cationic or ionizable lipids will first interact with nucleic acids. The intermediate nanostructures will make the hydrophobic tails available to interact with both structural lipids and modulators. The LNP structure will then be finalized by the formation of full functional vesicles able to both protect and deliver nucleic acids (Fig 1.).
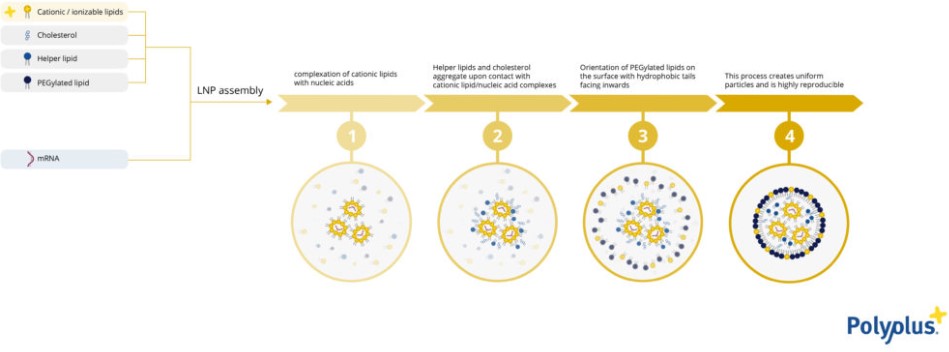
Figure 1: LNP formulation using microfluidic systems.
A deep understanding of the formulation steps, and of the microfluidic mixing, leads to self-assemble of the lipids and nucleic acids into the desired structure, finally resulting in scalable, reproducible and high-quality LNPs production.
