Mechanistic Modeling To Optimize rAAV Production For Gene Therapy
By Francesco Destro and John Joseph, MIT
Gene therapy has the potential to treat severe diseases by delivering therapeutic genes to the patient’s cells. Recombinant adeno-associated virus (rAAV) is the most popular vector for in vivo gene delivery, due to its relatively low immunogenicity, broad tissue tropism, and long-term gene expression capabilities. Recently, FDA has approved an increasing number of rAAV-based gene therapies for the treatment of various conditions, including genetic diseases that result in blindness, muscular dystrophy, and blood disorders.
Reproduced with author permission from Molecular Therapy – Methods & Clinical Development.
Manufacturing represents a substantial challenge in rAAV-based gene therapy, limiting the non-clinical and clinical development of new therapies. Current processes for rAAV manufacturing are generally not readily scalable and yield low vector titers with a high cost of consumables. As a result, the production costs (cost of goods sold, COGS) of a single dose an rAAV-based gene therapy can range from $100,000 to 300,000. This economic burden impedes access to high-quality, research-grade rAAV, significantly impacting the development of new rAAV-based gene therapies. At the same time, the current manufacturing capabilities are insufficient to meet the increasing demand for rAAV, especially considering the rising interest in systemically administered vectors, along with the hundreds of ongoing clinical and non-clinical studies for rAAV-based therapies. Therefore, there is a pressing need to develop efficient and scalable manufacturing processes to deliver rAAV-based gene therapies to patients.
There are two established and widely used rAAV manufacturing platforms. The most common technology for rAAV manufacturing is based on transient transfection of mammalian cells. Although fast to implement, transient transfection presents a high intrinsic cost for supplying plasmid DNA to the cells. Additionally, typically only a small percentage (5%-20%) of the rAAV produced through this approach is filled with the therapeutic gene. The rest of the rAAVs consist of empty capsids with no therapeutic effect, which is therefore considered a product impurity. In most cases, expensive processing steps to enrich the filled particles are needed downstream before administering the vector to the patient.
The second established production platform is based on the baculovirus expression vector system (BEVS) for rAAV manufacturing in the invertebrate Sf9 cell line. This process has emerged as a scalable and efficient alternative to HEK293 transient transfection, yielding high vector titers with up to 80% of filled rAAV capsids. This approach uses self-amplifying recombinant baculoviruses to deliver the genes for rAAV production to insect cells, representing a more cost-effective alternative to transient transfection. Notably, FDA recently approved for the first time an rAAV-based gene therapy manufactured with the BEVS using insect cells.
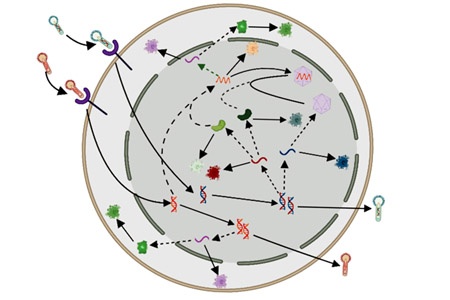
Several open questions need to be addressed to further enhance rAAV production with the BEVS. From a process perspective, the optimal cell density and baculovirus-to-cell ratio (multiplicity of infection, MOI) at the time of infection needs to be identified. From a molecular biology standpoint, it is unclear which genetic elements and pathways are responsible for the enhanced productivity of insect cell-based processes in comparison to the mammalian cell-based approach. Multiple baculovirus constructs, employing different promoters and recombinant cassettes, have been employed for rAAV production in insect cells. These diverse baculovirus constructs have exhibited varying efficiencies in terms of rAAV titer and filled-to-empty capsid ratio. An increased understanding of the intracellular mechanisms leading to higher titers of filled rAAV could be leveraged to engineer a baculovirus construct for enhanced rAAV production.

Adapted from Molecular Therapy – Methods & Clinical Development.
To accomplish these goals, a group of scientists and engineers at MIT collaborated with the team at UMass Chan Medical School that pioneered rAAV production using the BEVS. In a recent publication,1 the researchers develop a mechanistic model that quantitatively describes the key intracellular phenomena occurring during baculovirus infection and rAAV virion production in insect cells. The model uses differential equations to mathematically represent the physical, chemical, and biological phenomena involved in the process. Mechanistic modeling requires less training data compared to alternative methods such as machine learning, which makes such modeling particularly useful in scenarios where data are limited and expensive to obtain.
After having been trained, the model demonstrated an extraordinary validation performance on data sets not used in the training procedure. The model accurately predicted the titer of filled and empty rAAV capsids, along with the key intermediates for rAAV production and baculovirus amplification. Therefore, the model represents a quantitative and interpretable summary of the available mechanistic knowledge on the process, which can be interrogated to identify and address bottlenecks limiting the efficiency of rAAV manufacturing with the BEVS.
A user-friendly implementation of the model has been developed and is available online. The platform allows users to simulate rAAV production in shake flasks or bioreactors in batch mode. To run a simulation, the user simply needs to select the conditions of the experiment of interest, including the type of baculovirus construct to be used (TwoBac or ThreeBac), the cell concentration at the time of infection, the multiplicity of infection for each type of baculovirus present in the system, and the intended batch duration. Optionally, the user can also modify the default parameters for certain phenomena that are more likely to vary from lab to lab, such as the cell growth or death kinetics. Then, the simulator carries out the experiment with the selected conditions in a virtual environment, providing results within a few seconds and at no cost. The generated results consist of the model estimation for the concentration of filled and empty rAAV capsids during the batch. Furthermore, the results generated by the model include the concentrations of several intracellular species that play a role in the formation of rAAV: vector genomes, mRNAs, empty capsids, and viral replication and structural proteins. The user can tailor the simulation settings to quickly explore different experimental scenarios. Through a suitable design of the virtual experiments, the user can determine the optimal conditions that maximize the production of filled rAAV capsids. by understanding how the cell density and the MOIs affect the manufacturing process.
Gaining insights into the levels of intracellular species such as vector genomes and replicative proteins is crucial for identifying bottlenecks in the cascade of reactions that occur within the cell during rAAV production. From the simulator output, the user can readily plot the concentration of several intracellular species intervening in the formation of rAAV. Interestingly, the simulator output shows that, for the considered baculovirus constructs, the concentration of empty capsids in cells producing rAAV is much larger than the concentration of free vector genomes available for encapsidation. Hence, the model indicates that, with current baculovirus constructs, low rAAV genome amplification dictated by low replication protein levels is the main factor that limits the production of filled rAAV capsid. This conclusion had never been achieved before in experimental studies, since high experimental costs have greatly limited the number of reports on experimental measurements of proteins and nucleic acids inside cells that are producing rAAV. The model also indicates that several phenomena hypothesized in the literature as limiting steps, such as low efficiency of baculovirus infection, are not the actual bottlenecks that impede AAV production. The simulator allows testing a wide range of conditions and hypotheses, which otherwise may not be practical or feasible to demonstrate in a laboratory setting. For instance, by varying the kinetic parameters of the model, the user can explore the effect on filled capsids productivity of the overexpression or the underexpression of several genes involved in rAAV production.

Image created using BioRender's Academic Subscription.
In summary, the mechanistic model provides a virtual representation of an experimental setup, allowing researchers to predict rAAV productivity for different process parameters and baculovirus constructs. This robust tool provides suggestions to overcome current bottlenecks, facilitate rapid decision-making, and optimize experimental designs to maximize rAAV production.
Acknowledgment
The authors gratefully acknowledge the U.S. Food and Drug Administration for supporting the research discussed in this article through contract 75F40121C00131.
Reference
- Destro, F., Joseph, J., Srinivasan, P., Kanter, J.M., Neufeld, C., Wolfrum, J.M., Barone, P.W., Springs, S.L., Sinskey, A.J., Cecchini, S., Kotin, R.M. and Braatz, R.D. (2023). Mechanistic modeling explains the production dynamics of recombinant adeno-associated virus with the baculovirus expression vector system. Mol Ther Methods Clin Dev, 30, 122-146.
 About The Authors:
About The Authors:
Francesco Destro is a postdoctoral associate at MIT. His research focuses on the use of mechanistic modeling and machine learning to design and optimize manufacturing systems for advanced biotherapeutics. He earned his Ph.D. in chemical engineering from University of Padova (Italy). He can be reached on LinkedIn or by email at destro@mit.edu.
 John Joseph is a research scientist at MIT. His research focuses on gene therapy using rAAV vectors. His research interests include biomedical devices, immunoengineering, and gene therapy. He can be reached by email at johnjp@mit.edu.
John Joseph is a research scientist at MIT. His research focuses on gene therapy using rAAV vectors. His research interests include biomedical devices, immunoengineering, and gene therapy. He can be reached by email at johnjp@mit.edu.
