LipidBrick® IM21.7c: Cationic Lipids For LNP Formulation

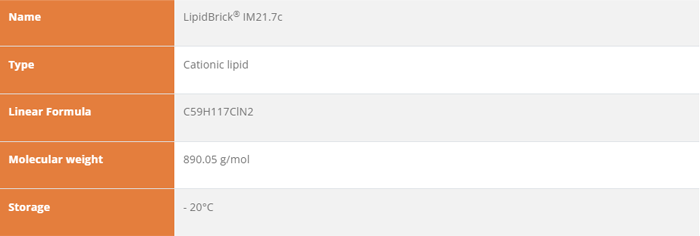
LipidBrick® IM21.7c
- Efficient: Modulates LNP properties to adapt the biodistribution depending on the therapeutic purpose
- Secure: Use a unique lipid structure protected by an independent patent owned by Polyplus®
- Accelerate your project: in vitro and in vivo proof of concept have been successfully performed
Summary
DNA and RNA therapeutics are drug products composed of an active genetic component linked to an efficient delivery system, generally Lipid NanoParticles – LNP. Those treatments include both prophylactic vaccines that prevent an infection by triggering the patient’s immune system and therapeutic vaccines that will be used to cure a patient from a specific disease. Each therapy will require its own LNP formulation depending on the genetic material and the targeted tissue. To adapt the delivery system to the need, a wide variety of lipids that modulate the encapsulation efficiency and the LNP properties is needed for therapeutic success.
As an innovator in the delivery field, Polyplus developed a range of cationic and ionizable lipids used in LNP formulation named LipidBrick®. Those “active lipids” will protect nucleic acids such as DNA, mRNA and siRNA and deliver them to targeted cells. By adding positive charges to an LNP, LipidBrick® broadens the current applications spectrum in terms of potency and targeting, as LNP electric charge is known to impact biodistribution and expression of mRNA-LNPs. LipidBrick® modifies the overall charge of the LNP, allowing to adapt the biodistribution.
Polyplus’ goal is to support customers from R&D to commercialization. LipidBrick® has been patented by Polyplus to avoid any patent infringement that could set back therapy advance.
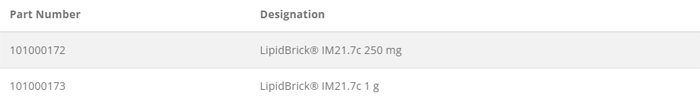
Ordering information