in vivo-jetRNA®+
Efficient: 100% mRNA encapsulation leading to comparable LNP delivery results
in vivo-jetRNA®+ is the reagent of choice to deliver mRNA to various organs. This is attributable to its intrinsic properties: in vivo-jetRNA®+ protects its payload against ubiquitous endonucleases, prevent non-specific interactions with proteins and promote efficient cell entry.
in vivo-jetRNA®+ shows an amazing capacity to encapsulate 100% of the mRNA available (Fig 1 A/) leading to an efficient gene expression that is comparable to lipid nanoparticles currently used as a gold standard for vaccination purpose (Fig 1 B/).
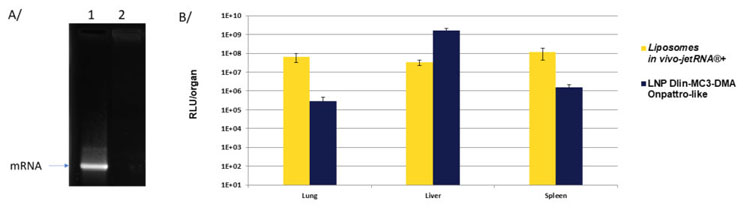
Fig1. in vivo-jetRNA®+ leads to 100% mRNA encapsulation leading to efficient mRNA delivery. A/ mRNA alone (lane 1) and mRNA fully encapsulated using in vivo-jetRNA®+ (lane 2) were analyzed by gel electrophoresis (agarose gel). B/ mRNA encoding Luciferase was injected into mice using in vivo-jetRNA®+ or Dlin-MC3-DMA LNP through intravenous injection. Liposomes were formed using 10 µg of mRNA with an mRNA/in vivo-jetRNA®+ ratio of 1:2 (µgmRNA:µLreagent) in mRNA Buffer. Luciferase expression was assessed 24 h post-injection.
Ready-to-use: Liposome-based reagent that does not require any formulation equipment
Lipid nanoparticles (LNP) are a gold standard to deliver mRNA but it requires months of work, dedicated equipment and consumables to reach the optimal and efficient formulation. With in vivo-jetRNA®+, there is no need to spend time and budget to reach the perfect formulation. in vivo-jetRNA®+ has been carefully optimized by Polyplus and can be used with a simple and straightforward protocol without any formulation step.
in vivo-jetRNA®+/mRNA liposome solution is prepared in two steps and ready to be injected to the animal in 15 minutes: a gain of time with minimal effort (Fig. 2). Contrary to LNP, in vivo-jetRNA®+ is a ready to use formulation that do not require any formulation equipment.
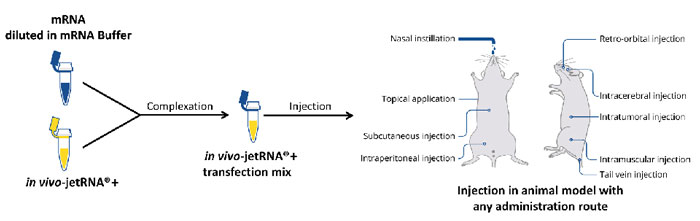
Fig 2. in vivo-jetRNA®+ simplified protocol. This two-step protocol is suitable with direct injection of in vivo-jetRNA®+/mRNA liposomes through any systemic or local administration route. Contrary to LNP, in vivo-jetRNA®+ is a ready to use formulation that does not require any formulation equipment.
Our friendly scientific support team use their scientific expertise to provide protocols tailored to meet your needs.
Stable: Liposomes are stable using low/high mRNA concentration
In addition of the capacity to encapsulate mRNA, in vivo-jetRNA®+ excels in stability when it is formulated with mRNA. Liposome’s sizes are stable up to 1 month when incubated at room temperature or 4°C using in vivo-jetRNA®+ (Fig 3. A/). The mRNA concentration will influence the liposome’s size but in vivo-jetRNA®+ shows a stable liposome size at low or high mRNA concentration (Fig 3. B/).
This makes in vivo-jetRNA®+ the reagent of choice for vaccine or mRNA cancer therapy.
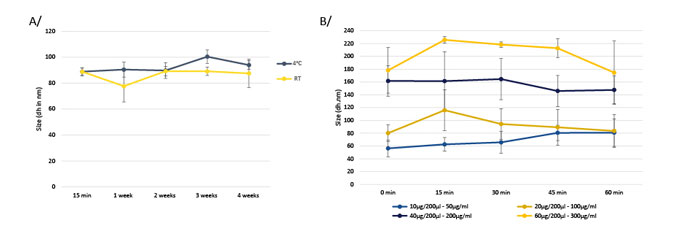
Fig 3. Liposomes (including in vivo-jetRNA®+ & mRNA) are very stable over the time using low to high mRNA concentration. A/ Size of liposomes with in vivo-jetRNA®+ at 50 μg/ml after 15 min, 1 week, 2 weeks, 3 weeks and 4 weeks of complexation stored at room temperature (RT) or 4°C were measured with the dynamic light scattering (DLS). B/ Size of liposomes with in vivo-jetRNA®+ at 50, 100, 200 or 300 μg of mRNA/ml with a small mRNA (1929b) were measured by DLS.
Universal: Target any organ/tissue using various administration routes
Choice of the administration route leads to different mRNA biodistribution. in vivo-jetRNA®+-mediated mRNA delivery leads to gene expression in numerous organs, through intraperitoneal injection, especially in the spleen and nodes that have a major role in the immune response but also in other organs such as lung, liver, pancreas and uterus (Fig. 4).
Being a non-viral delivery reagent, in vivo-jetRNA®+ can be safely administered in animals. By avoiding conventional vaccine approaches, it allows to generate a new class of vaccines that rely on direct gene transfer. This concept has several advantages – it is safer for the recipients, faster and cheaper to produce compared to viral vector vaccines or peptide loaded APC (Antigen Presenting Cells), and it has an immense potential to tackle many unmet medical needs.
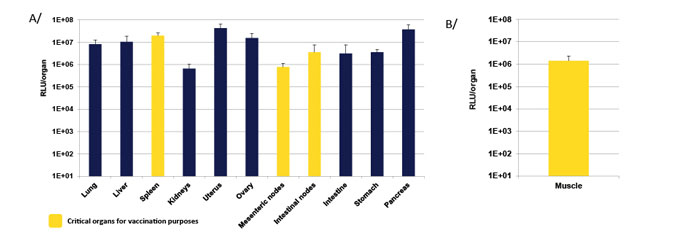
Fig 4. in vivo-jetRNA®+ leads to efficient mRNA delivery in different organs depending on the administration route. mRNA encoding Luciferase was injected into mice using in vivo-jetRNA®+ through different administration routes A/ intraperitoneal (IP) and B/ intramuscular (IM). Liposomes were formed with a mRNA/in vivo-jetRNA®+ ratio of 1:2 (µgmRNA:µLreagent) in mRNA Buffer using either 20 µg mRNA for intraperitoneal (IP) injection or 5 µg mRNA for intramuscular (IM) injection. Luciferase expression was assessed 24 h post-injection.
Safe: Maintain healthy animals and organs
in vivo-jetRNA®+ is the best choice in terms of safety. There are no secondary effects after injection, all animals remain healthy and no animal pain has been encountered. After administration of mRNA – in vivo-jetRNA®+ liposomes by any administration route, targeted organ remains phenotypically intact and no tissue damage is observed.
Furthermore, using in vivo-jetRNA®+ triggers no pro-inflammatory cytokine expression (Fig 5.)
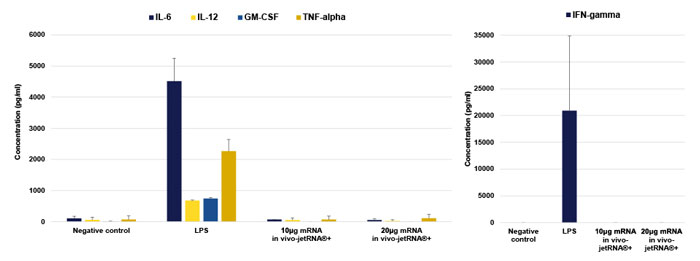
Fig. 5 in vivo-jetRNA®+ triggers no pro-inflammatory cytokine expression. mRNA liposomes were formed in 200 µL of mRNA Buffer using 10 or 20 µg of mRNA encoding Luciferase at a mRNA/in vivo-jetRNA®+ ratio of 1:2 (µgmRNA:µLreagent) and injected through intravenous injection (retro-orbital injection). 2 to 24 hours after injection, blood was collected and the level of IL-6, IL-12, GM-CSF, IFN-gamma and TNF-alpha was measured by ELISA (IL-6) or MACSPlex kits. As a positive control, LPS (200 µg) was administered into mice.
