Immune Checkpoint Inhibitors Global Clinical Trials Landscape (2023)
Immunotherapy, a groundbreaking cancer treatment approach, has gained prominence with the advent of immune checkpoint inhibitors. These inhibitors harness the body's immune system to combat cancer, provi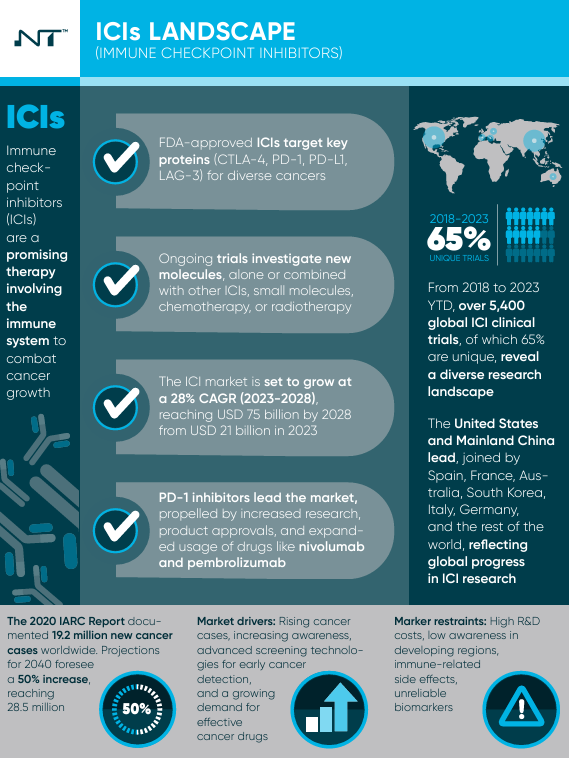 ng effective both independently and in conjunction with traditional methods such as chemotherapy and radiation.
ng effective both independently and in conjunction with traditional methods such as chemotherapy and radiation.
The FDA's approval of inhibitors targeting proteins like CTLA 4, PD 1, PD L1, and the more recent LAG 3 marks a pivotal milestone in cancer treatment, opening avenues for novel therapeutic interventions. An extensive analysis spanning 2018 to 2023 reveals a diverse research landscape with over 5,400 immune checkpoint inhibitor trials globally. The Asia Pacific region, notably Mainland China, plays a leading role, contributing to 36% of trials, emphasizing its increasing importance in immunotherapy development. Regional variations in patient recruitment highlight the need for tailored approaches in clinical trial planning.
Examining the clinical trial landscape reveals additional intriguing patterns, with 65% of studies being unique, reflecting a complex research environment dedicated to addressing specific issues in immunotherapy. A SWOT analysis uncovers strengths, weaknesses, opportunities, and threats in immune checkpoint inhibitor-based immunotherapy. While efficacy spans various malignancies, challenges include costs and limited effectiveness in certain patient categories. Opportunities lie in combination therapies and overcoming resistance, while threats encompass competition from other immunotherapies and regulatory hurdles.
In conclusion, immune checkpoint inhibitors offer hope in cancer treatment, and ongoing research and collaboration promise substantial advancements. Stakeholders seeking detailed insights can access the whitepaper for a comprehensive overview of the current state and future directions of immune checkpoint inhibitors-based immunotherapy.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
