High Titer Lentivirus Vector Production

Achieve high titers in lentivirus vector production, ensuring efficient and reliable results for your research or therapeutic applications.
These advanced reagents and protocols are designed to support every step of your process, from initial research to large-scale production.
Performance and Platform Flexibility
Higher Titers than Other Reagents
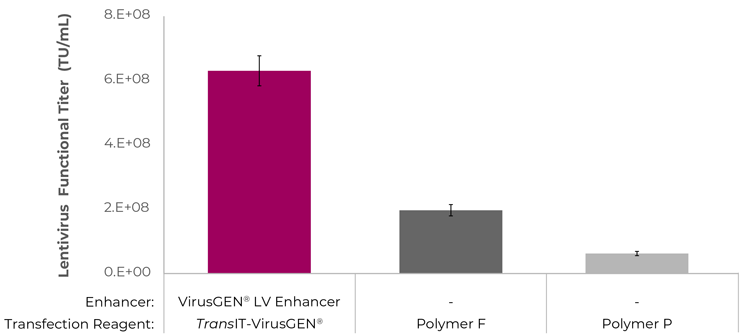
TransIT-VirusGEN® is superior to competitor reagents in suspension lentivirus cell cultures.
Higher Efficiency than PEI
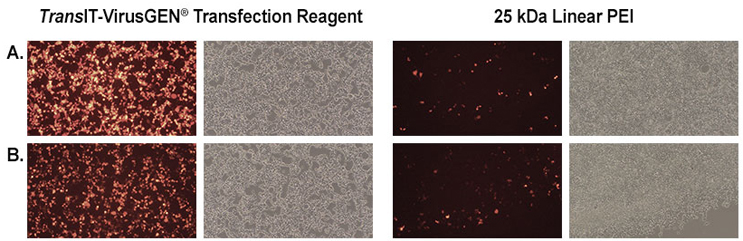
HEK293T/17 cells were transfected with lentiviral packaging plasmids and either (a) control RFP transfer plasmid or (b) targeting SPdCas9-RFP transfer plasmids using either TransIT-VirusGEN® Transfection Reagent or 25 kDa linear PEI. Data courtesy of Zuzana Drobna, PhD (Dr. Keung’s Lab), Department of Chemical and Biomolecular Engineering, North Carolina State University.
Works with different packaging and transfer plasmids
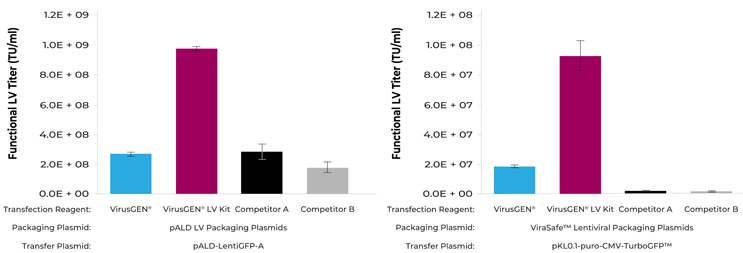
The TransIT-VirusGEN® LV Kit consistently outperforms competitor reagents, even with different packaging systems. LVV packaging systems greatly influence functional titer.
Works Across 293 Suspension Cell and Media Platforms

The TransIT-VirusGEN® LV reagents and kits are compatible with multiple cell lines and media formulations.
Lentivirus Production Protocol
Take advantage of top-of-industry support to start your research faster, or bring it to commercialization sooner and more efficiently. Work with a dedicated Field Application Scientist from our support team, explore the citation search tool, or peruse the recommended protocols
Overcoming Lentivirus Challenges
 Utilize the most responsive support team in the industry. Our team of transfection experts is dedicated to helping you succeed.
Utilize the most responsive support team in the industry. Our team of transfection experts is dedicated to helping you succeed.
A committed team of Field Application Scientists (FAS) can help you plan your experiments, review your data, and provide on-site assistance to set up your research or manufacturing runs, saving you time and resources.
Personalized support to optimize your process, reducing the risk of bioreactor issues, wasted materials, lost time, or wasted budget.
GMP Lentivirus Production
Your drug assets require the support of GMP and ISO-compliant material, designed to support the commercial-scale production. Choose reagents and enhancers that maintain their results from the smallest shake flasks to the largest bioreactors.
Confidently move your lentivirus production from the lab to commercial manufacturing.
