Gene Therapy & AADC Deficiency
By Irina A. Anselm, MD, Director of the Mitochondrial Program and Co-Director of the Neurometabolic Program at Boston Children's Hospital
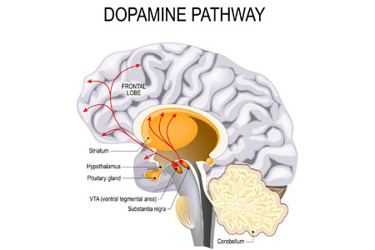
Innovations in the field of gene therapy now aim to treat rare diseases, including aromatic L-amino acid decarboxylase (AADC) deficiency. AADC deficiency causes severe disease burden, manifesting in physical, mental, and behavioral symptoms from the first few months of life.1, 2, 3 There are currently no effective treatment options for this rare and extremely devastating disease.
With global experts in the United States, Taiwan, France, Germany, and Japan, I co-authored a manuscript recently published in the European Molecular Biology Organization Journal (EMVO). The paper describes three clinical trials in which a novel gene therapy, PTC-AADC, discovered and developed by biopharma company PTC Therapeutics, was infused via brain surgery into the putamen of 20 children with AADC deficiency. These trials demonstrate that surgical delivery of PTC-AADC directly to the putamen successfully and robustly results in dopamine production and leads to substantial and sustained functional improvements.
Addressing the Unmet Need with Gene Therapy
Dopamine is a neurotransmitter critical for motor and mental development.1 AADC deficiency typically presents in infancy and is characterized biochemically by deficiency of certain neurotransmitters and monoamines, including dopamine, epinephrine, norepinephrine, and serotonin. Patients experience severe long-term physical and intellectual disability and face risk of premature death from complications related to the disease.
In AADC deficiency, dopamine is not produced in the part of the brain called the putamen, which is, therefore, a prime target for gene therapy.
While patients with AADC deficiency develop symptoms soon after birth, the diagnosis is very frequently delayed and, in many cases, missed. All patients have abnormal muscle tone, with combination of rigidity and low muscle tone (hypotonia). They suffer from painful muscle spasms (dystonic crises); oculogyric crises (abnormal eye movements), fluctuations of blood pressure, heart rate, and body temperature; and emotional lability. Almost all patients are unable to sit, stand and walk. Their language is also severely affected. There is currently no cure for the disease, and symptomatic therapies bring only minimal relief. Thus, the importance of gene therapy cannot be overstated.
How Does PTC-AADC Work?
The goal of PTC-AADC is to restore production of neurotransmitters, especially dopamine, in the areas of the brain, where it is severely depleted. A functional copy of the human dopa decarboxylase (DDC) gene, critical to dopamine production, is infused directly into the brain utilizing low doses of adeno-associated viral vectors.1
To evaluate the safety and efficacy of PTC-AADC, this gene therapy was surgically administered directly into the putamen on each side. Putamina is relatively more easily accessible via surgery than other potential sites, such as midbrain, and therefore, these infusions may result in fewer surgical complications.1 Every child participating in one of the three trials showed progress following treatment with PTC-AADC, with improvements in motor function and development. Dystonic and oculogyric crises resolved. There was improvement in autonomic function and emotional status. Efficacy was further supported by positron emission tomography (PET) and CSF analysis, that showed increased dopamine and serotonin metabolites.
There were no complications associated with PTC-AADC.
The clinical benefits and safety profile of PTC-AADC have been demonstrated across several trials, with the first patient dosed in 2010. The above published report summarizes data on the largest cohort of AADC deficiency patients that received gene therapy.
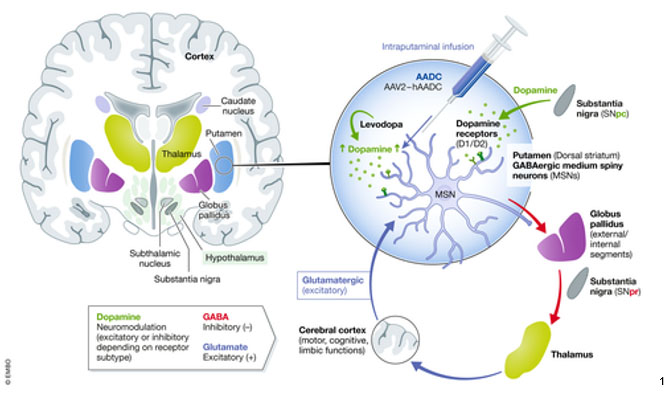
Simplified circuit diagram illustrating the key afferent and efferent connections of the dorsal striatum (putamen) and their role in the control of the motor, cognitive, and limbic functions.
The Impact
The data reported in the EMBO publication demonstrate that the surgical approach delivering PTC-AADC gene therapy directly to the putamen produces dopamine in the brain, resulting in sustained and substantial functional improvements in children with AADC deficiency.
Irina A. Anselm, MD
 Irina A. Anselm, MD, is Director of the Mitochondrial Program and Co-Director of the Neurometabolic Program at Boston Children's Hospital. A pediatric neurologist interested in genetics and hereditary disorders, she cares for children with neurometabolic, neurodegenerative, and mitochondrial disorders. She serves as the Department of Neurology's clinical expert for Boston Children's Precision Medicine Service. Her research focuses on the genetics, diagnosis, and management of these disorders, which range from mild to devastating. She is a co-investigator on a multicenter trial for the treatment of patients with mitochondrial disorders with intractable seizures. She also is a co-investigator on a natural history study of patients with creatine transporter deficiency. She has a special interest in disorders of neurotransmitter metabolism and works closely with a company that developed gene therapy for one of these disorders. Major publications include 40 original reports in peer-reviewed journals and four chapters, and she is a reviewer for the Journal of Pediatric Neurology, Current Pediatric Reviews, and the Journal of Child Neurology.
Irina A. Anselm, MD, is Director of the Mitochondrial Program and Co-Director of the Neurometabolic Program at Boston Children's Hospital. A pediatric neurologist interested in genetics and hereditary disorders, she cares for children with neurometabolic, neurodegenerative, and mitochondrial disorders. She serves as the Department of Neurology's clinical expert for Boston Children's Precision Medicine Service. Her research focuses on the genetics, diagnosis, and management of these disorders, which range from mild to devastating. She is a co-investigator on a multicenter trial for the treatment of patients with mitochondrial disorders with intractable seizures. She also is a co-investigator on a natural history study of patients with creatine transporter deficiency. She has a special interest in disorders of neurotransmitter metabolism and works closely with a company that developed gene therapy for one of these disorders. Major publications include 40 original reports in peer-reviewed journals and four chapters, and she is a reviewer for the Journal of Pediatric Neurology, Current Pediatric Reviews, and the Journal of Child Neurology.
1 Hwu WL, Kiening K, Anselm I et al. Gene Therapy in the Putamen for Curing AADC Deficiency and Parkinson Disease'. EMBO Molec Medicine. 2021. DOI 10.15252/emmm.202114712. Available at: https://www.embopress.org/doi/10.15252/emmm.202114712. Last accessed August 2021.
2 Wassenberg T, et al. Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis. 2017;12(1):12.
3 Williams K et al. Symptoms and impacts of aromatic l-amino decarboxylase (AADC) deficiency: A qualitative study. Poster presented at ISPOR 2021, May 17-20, 2021
