From Open Cleanrooms To Closed Systems – What Is Driving The Change?
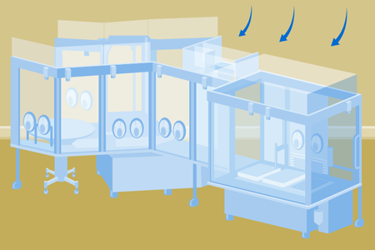
Traditionally, aseptic processing has been conducted in open cleanroom environments, relying on air filtration and extensive gowning to minimize contamination. However, the inherent risk of human contamination has led to regulatory bodies advocating for the segregation of personnel from critical processing zones.
This white paper explores the driving forces behind this shift, examining the increasing emphasis on closed systems and barrier technologies like Restricted Access Barrier Systems (RABS) and isolators. It delves into the regulatory perspectives, particularly the impact of documents like GMP Annex 1, which outlines the expectations for minimizing contamination risk in aseptic operations. Understanding these changes is crucial for any organization involved in sterile drug production.
Download this white paper to gain insights into how regulations and technological advancements are shaping the future of aseptic processing.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
