Excellence For Cell And Gene Therapy Manufacturing
Unlock the potential of advanced therapies with dedicated partnership and tailored manufacturing solutions, ensuring quality and efficiency from discovery through to delivery for next-generation treatments.
Cell and gene therapies have the potential to revolutionize healthcare by offering unprecedented treatment possibilities.* At Roche CustomBiotech, we understand the technical complexity involved in implementing transformative advanced therapy medicinal products (ATMPs). Our commitment is to be your dedicated partner in providing tailored solutions for manufacturing advanced therapeutics.
By utilizing precisely defined and scalable raw materials, coupled with robust quality control solutions, we support manufacturing efficiency and compliance with regulatory requirements. Located in our state-of-the-art biotechnology facility in Penzberg, Germany, we combine the expertise of pharma and diagnostics with decades of experience in producing high-quality raw materials. Join us in advancing cell and gene therapies from cell isolation to quality control release testing.
Available now: EndoCleave - Roche’s endonuclease for viral vector manufacturing
Learn more
Our commitment to next-generation manufacturers: high-quality, well-characterized raw materials
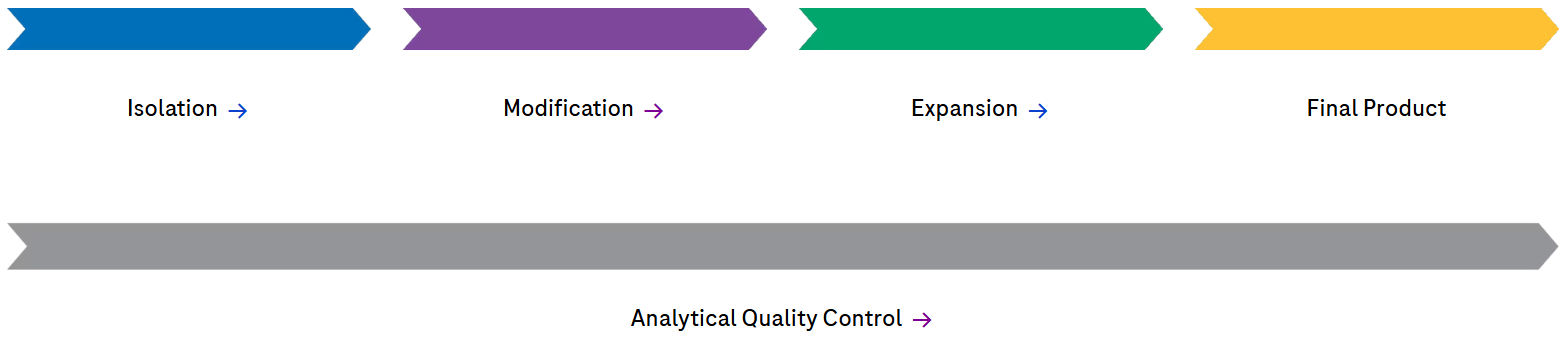
Isolation enzymes
Maximize yields of viable cells with efficient cell isolation
High-purity enzymes with well-characterized performance parameters are essential for safe and efficient cell isolation from diverse tissue sources. Our collagenases ensure maximum cell yield, while DNase I allows for elimination of interfering DNA. Collectively, our enzymes ensure high cell yield and viability and thus optimal downstream applications.
Modification: mRNA raw materials
Leverage our experience with fit-for-purpose & GMP Grade mRNA reagents
At Roche CustomBiotech, we strive to enhance our mRNA raw materials portfolio to meet the evolving needs of cell and gene therapy developers and manufacturers. Produced under GMP Grade and ISO 13485:2016 quality standards*, our materials support innovative drug development, including CAR-T cell therapy and CRISPR/Cas9 applications.
*exceptions apply, please see overview table on mRNA reagents portfolio page
Expansion: Cell detachment enzymes
Gain certainty with Trypsin, rec., GMP Grade
Fast, effective, yet gentle detachment of cells is vital for scaling manufacturing of cell and gene therapies. Trypsin, a serine protease, efficiently facilitates cell detachment by cleaving peptide bonds, enabling rapid separation of cells from culture surfaces. Simultaneously, the final product must be safeguarded against variability or contamination introduced by raw materials like enzymes.
Analytical Quality Control
![arrow]() Cedex Analyzers
Cedex Analyzers
Take control of your cell and gene therapy bioprocess with Cedex® Analyzers
In the development of cell and gene therapies, effective bioprocess control is crucial for a seamless transition to commercialization. Close monitoring of culture conditions ensures consistency, quality, and predictable outcomes. Implementing validated commercial technologies overcomes the challenge of diverse starting materials and processes.
Mycoplasma testing
Deliver constant product quality with rapid and reliable quality control kits
Fast, sensitive and reliable quality control kits facilitate manufacturing with constant product quality. With our QC Sample Preparation kit, efficient sample preparation for subsequent PCR tests is ensured. The MycoTOOL Real-Time PCR Kit allows for sensitive mycoplasma detection, which is crucial in manufacturing workflows to ensure the quality and safety of cell-based medicinal products. Additionally, the residual DNA E. coli kit enables the accurate quantification of residual host cell DNA, thereby further enhancing the overall manufacturing reliability.


 Cedex Analyzers
Cedex Analyzers