Developing A Therapeutic Platform To Enable Immunotherapy For Liver Tumors
By Steven C. Katz, MD, FACS, FSSO, Chief Medical Officer, TriSalus Life Sciences
Immunotherapy has enabled remarkable improvements in outcomes for patients with hematologic malignancies and a small number of solid tumor indications. While checkpoint inhibitor therapy has prolonged survival in advanced cutaneous melanoma and non-small cell lung cancer,1 the adoption of immunotherapy for primary and metastatic liver tumors has thus far been limited.2 The deep and durable responses achieved in certain indications with immunotherapies have largely eluded those patients suffering with liver tumors.
small number of solid tumor indications. While checkpoint inhibitor therapy has prolonged survival in advanced cutaneous melanoma and non-small cell lung cancer,1 the adoption of immunotherapy for primary and metastatic liver tumors has thus far been limited.2 The deep and durable responses achieved in certain indications with immunotherapies have largely eluded those patients suffering with liver tumors.
Given the lack of consistent and meaningful success for patients with liver tumors, we must ask ourselves if there are any barriers inherent to the intrahepatic space. Delivery of therapeutics into high-pressure liver tumors is challenging via conventional approaches and formidable immunosuppressive barriers in the liver limit therapeutic success.3 A multi-faceted approach focused on enhancing delivery and intrahepatic performance of immunotherapeutics may be required to address this patient population.
Suppression of Anti-tumor Immunity in the Liver
Tumor cells actively influence their microenvironment to drive immunosuppression and promote a hospitable niche for progression of disease.4 The liver is skewed toward tolerance at baseline, which may exacerbate the suppressive tendencies of intrahepatic malignancies. Tumors not only evade immune responses induced by therapeutic agents, but also actively produce immunosuppressive factors that promote tumor growth and prevent eradication.3 As such, effective immunotherapy for liver tumors may require not only effective tumor targeting, but also therapies capable of limiting intrahepatic immunosuppression.
The immunologic landscape varies greatly among different organs which can have a biologic impact on cancer progression and response to treatment. Cancer drugs traditionally target specific histologic subtypes, such as colorectal, breast or pancreatic cancer, irrespective of the tumor location. Requirements for effective treatment of liver metastases with immunotherapy may be quite different than for lung metastases for example, even when arising from the same primary tumor. The liver is a unique organ with an intrinsic immunosuppressive environment that is exacerbated in the setting of malignancy by myeloid derived suppressor cells (MDSC) and a variety of other cell types.3
MDSCs, a population of bone marrow-derived myeloid progenitors, are pro-tumorigenic and are present in large numbers in solid malignancies.5 The liver can also induce suppressive programming among T cells, including induction of regulatory T cells (Treg) and immunosuppressive cytokine production from intrahepatic T lymphocytes.6 This unique feature is beneficial for liver transplantation, but is a major hindrance for immunotherapy treatment of liver tumors.
Solid Tumor Drug Delivery Barriers
Systemic delivery of cancer therapeutics presents two critical challenges for patients with liver tumors. Firstly, based on the normal distribution of cardiac output, the liver will receive a fraction of the dose.7 The second challenge, high intratumoral pressure, is an underappreciated but important barrier to effective drug delivery into liver metastases. Intratumoral solid stress and interstitial fluid pressure, which are primary contributors to intratumoral pressure, may compress the interior of the tumor and deform blood vessels, reducing the delivery of therapeutics.8 As such, a technological solution to the intratumoral pressure barrier may enable more effective delivery of therapeutic agents to liver tumors.
Pressure-Enabled Drug Delivery™ (PEDD™) devices are engineered to overcome high intratumoral pressure through creation of a favorable pressure gradient, while at the same time helping to reduce off-target organ exposure through anti-reflux properties.9,10 This has been shown to overcome the infusion barriers of the tumor microenvironment and improve therapy penetration into liver tumors.11 The capability of PEDD to modulate pressure and improve therapeutic delivery has also been demonstrated in the pancreas, further validating the PEDD approach.12
PEDD is currently utilized as part of routine clinical care for patients with primary and metastatic liver tumors. In a retrospective study comparing PEDD (n=18) to standard catheters (n=70) for hepatic arterial infusion (HAI) for chemoembolization therapy in hepatocellular carcinoma patients in the pre-transplant setting, response rates were significantly higher (100% vs. 76.5%, p = 0.019).11 PEDD resulted in greater concentrations of microspheres within the tumor relative to the surrounding tissue (p=0.002) and there was significantly higher percentage of tumor necrosis in the PEDD group (p=0.006) (PEDD n=4, standard catheter n=12).
PEDD has also shown promise with the use of immunotherapy for liver metastases (LM) from pancreatic ductal adenocarcinoma. PEDD enhanced chimeric antigen receptor T cell (CAR-T) delivery to LM 5.2‑fold compared to standard catheter infusion.13 Three doses of anti-CEA CAR-T were regionally delivered via PEDD/HAI with systemic IL-2 to support CAR-T in vivo were reported in a single patient with pancreas LM.14 HAI of anti-CEA CAR-T was not associated with any severe adverse events (SAEs) above grade 3 and there were no on-target/off-tumor SAEs. Following CAR-T PEDD/HAI treatment, positron emission tomography-CT demonstrated a complete metabolic response within the liver, which was durable and sustained for 13 months. The response was accompanied by normalization of serum tumor markers and an abundance of CAR+ cells found within post-treatment tumor specimens. These findings demonstrated the potential of PEDD/HAI to optimize the therapeutic index of immuno-oncology therapies for LM.
Creating a Therapeutic Platform for Liver Tumors
As noted above, the barrier to therapeutic success posed by delivery challenges in liver tumors is accompanied by the potent immunosuppression present in the intrahepatic space. One strategy for stimulation of the immune system is through administration of toll-like receptor agonists (TLRA).15 TLRAs are present on a variety of immune cells through the body, with their primary function being to trigger initial, non-specific immune responses to danger signals. Such danger signals include nucleic acid motifs released for bacteria or viruses. TLRAs are designed to simulate naturally occurring danger signals, with the capacity to stimulate a broad array of immune cells. There are multiple TLR types, and TLR9 is a variant that has been the target of many immunotherapy programs.
Activation of TLR9 has been shown to have anti-cancer effects in animal models, and promising clinical activity in cutaneous melanoma and other malignancies has been reported in combination with checkpoint blockade.16 DNA containing unmethylated dinucleotide CpG motifs, which make up TLR9 agonists, are common in the DNA of viruses and bacterial genomes.17 Stimulation of TLR9 by CpG oligodeoxynucleotides (CpG-ODNs), initiates a variety of innate and adaptive immune responses.15 What is less appreciated is the expression of TLR9 by immune cells in the liver, including MDSC.18,19 Furthermore, MDSC are abundant in the setting of liver metastases and suppress both endogenous immunity and activity of immune-oncology therapies.20
Figure - Mechanisms of Therapeutic Activity of TLR9 Agonists
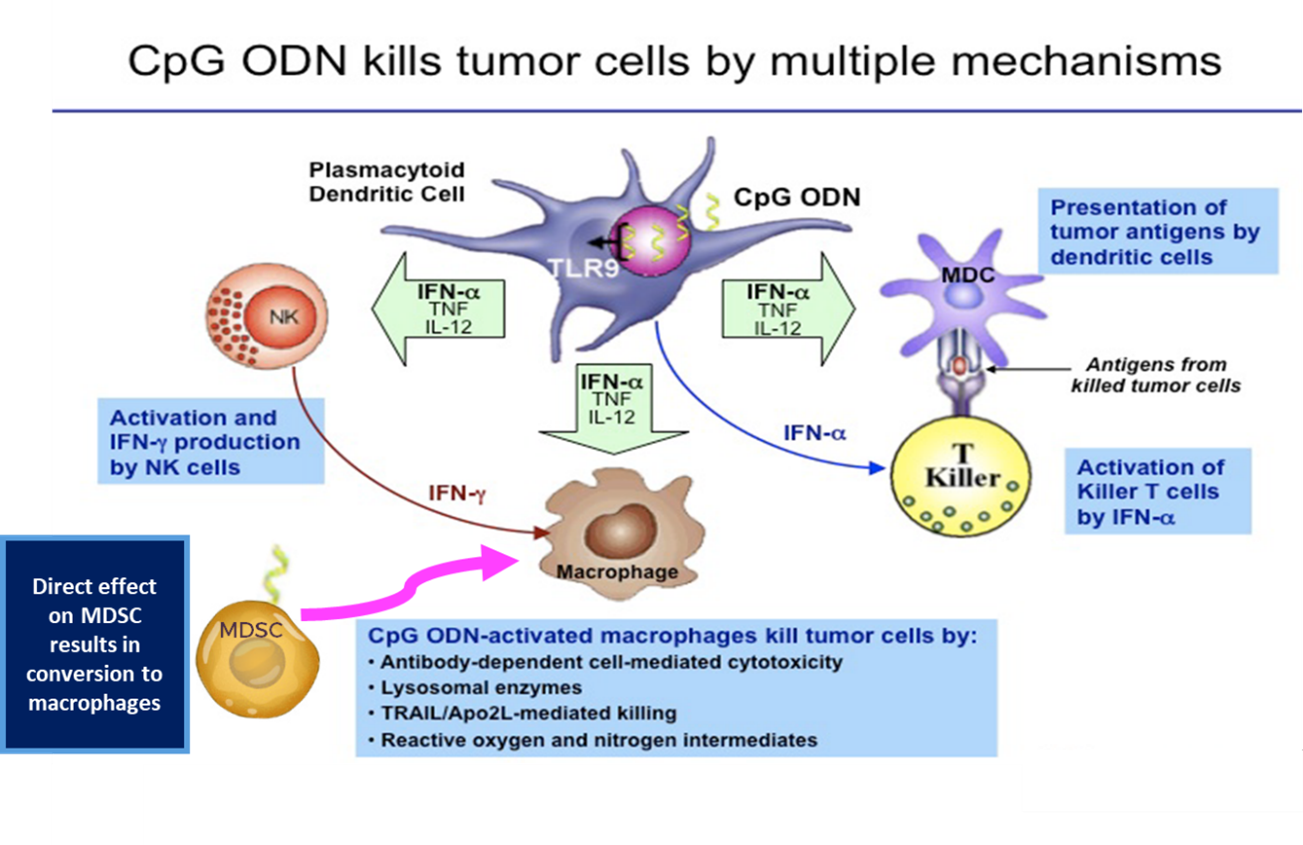
TLR9 agonists have shown clinical activity and have been well tolerated when delivered by direct needle injection into superficially located tumors.21 Systemic delivery of TLR9 agonists can be challenging and direct needle injection is problematic in patients with multiple, deep liver tumors. A therapeutic platform consisting of hepatic arterial infusion of a TLR9 agonist via PEDD is an intriguing concept for enhancing response rates to immunotherapy in patients with liver tumors.
Ongoing Challenges
Cancer immunotherapy has evolved significantly, from the time of Coley’s observations of a spontaneous sarcoma regression to the recent clinical testing of immune checkpoint blockade and genetically modified T cells. Our increased understanding of immunobiology and immunosuppressive pathways has facilitated translation of animal work into groundbreaking clinical trials. For patients with liver tumors resistant to immunotherapy, innovative delivery approaches coupled with strategic immunostimulants may enable deep responses. As we study novel combinatorial clinical approaches, we hope that an increasing number of liver cancer patients will benefit from innovative immunotherapies.
- Alsaab, H. O. et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 8, 561 (2017).
- Dai, X., Wang, S., Niu, C., Ji, B. & Liu, Y. Overview of Current Progress in Immune Checkpoint Inhibitor Therapy for Advanced Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 19, 1533033820947486 (2020).
- Guha, P., Reha, J. & Katz, S. C. Immunosuppression in liver tumors: opening the portal to effective immunotherapy. Cancer Gene Ther. 24, 114–120 (2017).
- Whiteside, T. L. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin. Cancer Biol. 16, 3–15 (2006).
- Jones, N. M., Yang, H., Zhang, Q., Morales-Tirado, V. M. & Grossniklaus, H. E. Natural killer cells and pigment epithelial-derived factor control the infiltrative and nodular growth of hepatic metastases in an Orthotopic murine model of ocular melanoma. BMC Cancer 19, 484 (2019).
- Shimizu, K., Iyoda, T., Okada, M., Yamasaki, S. & Fujii, S. Immune suppression and reversal of the suppressive tumor microenvironment. Int. Immunol. 30, 445–455 (2018).
- Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
- Stylianopoulos, T. et al. Coevolution of Solid Stress and Interstitial Fluid Pressure in Tumors During Progression: Implications for Vascular Collapse. Cancer Res. 73, 3833–3841 (2013).
- Data on file, T. L. S. CEA-001 Clinical Trial. (2019).
- Fischman, A. M. et al. Prospective, Randomized Study of Coil Embolization versus Surefire Infusion System during Yttrium-90 Radioembolization with Resin Microspheres. J. Vasc. Interv. Radiol. 25, 1709–1716 (2014).
- Titano, J. J. et al. End-hole Versus Microvalve Infusion Catheters in Patients Undergoing Drug-Eluting Microspheres-TACE for Solitary Hepatocellular Carcinoma Tumors: A Retrospective Analysis. Cardiovasc. Intervent. Radiol. 42, 560–568 (2019).
- Shankara Narayanan, J. S. et al. Pressure-enabled delivery of gemcitabine in an orthotopic pancreatic cancer mouse model. Surgery 168, 448–456 (2020).
- Hardaway, J. C. et al. Pressure enabled delivery of CAR-T cells into porcine pancreas resulted in highly targeted delivery with minimal systemic exposure and no pancreatitis or systemic cytokine release. Soc. Immunother. Cancer 33, 1 (2018).
- Katz, S. C. et al. HITM-SURE: Hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J. Immunother. Cancer 8, e001097 (2020).
- Krieg, A. M. Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 117, 1184–1194 (2007).
- Sato-Kaneko, F. et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2,.
- Krieg, A. M. CpG Motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 20, 709–760 (2002).
- Hossain, D. M. S. et al. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 21, 3771–3782 (2015).
- Shirota, B. Y., Shirota, H. & Klinman, D. M. Intra-tumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J. Immunol. Baltim. Md 1950 188, 1592–1599 (2012).
- Medina-Echeverz, J., Eggert, T., Han, M. & Greten, T. F. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol. Immunother. CII 64, 931–940 (2015).
- Brody, J. D. et al. In Situ Vaccination With a TLR9 Agonist Induces Systemic Lymphoma Regression: A Phase I/II Study. J. Clin. Oncol. 28, 4324–4332 (2010).
