Defining Data Driven, Foundational Model For Right First Time CGT Manufacturing At Scale
By Sanjay Srivastava, Ph.D., Managing Director, Cell & Gene Therapy CoE Lead; Marcus Carpentier, Life Sciences / Cell & Gene Therapy SME; and Martha Perez Linares, Management Consultant, Life Sciences SC&O / Cell & Gene Therapy at Accenture

Cell and gene therapy (CGT) has evolved in the past several years and has provided breakthrough treatments to patients all over the world. Like no other treatment modalities before them, CGT therapies are greatly impacting patient lives. However, to date, all commercial product launches have experienced significant operational challenges. This is in part because traditional drug development approaches do not take into account the cell biology aspect required to efficiently manufacture and commercialize these therapies. Attempts to resolve the operational challenges post-launch is like reworking the foundation after a house is built – it is not efficient. Emerging CGT companies can adopt a new business model, embracing a data-driven culture and risk-based approach to pro-actively prevent known future roadblocks. Leveraging a data-driven culture and risk-based approach at each development phase will introduce “foundational” manufacturing strategies that embed right first time risk reduction capabilities for patients as the process matures.
Introduction



What’s the difficulty?
Although the strong potential of CGT is evident from clinical trials data, companies continue to face operational challenges post-FDA approval, and are unable to efficiently ramp up and optimize the manufacturing processes. It is critical to understand these common roadblocks to commercializing CGTs. While product launches will differ by each company and for each CGT modality, our experience indicates that there are three common operational challenges that can be addressed (Figure 1).

Figure 1. CGT Manufacturing Challenges
1. Complex Supply Chain
- Effective Demand Planning: CGT related supply chain and manufacturing challenges are new to the industry. Considering the patient-specific variability aspects (specifically for the autologous cell therapies), effective demand planning and manufacturing are crucial; once considered an enabling function, has come to the forefront, requiring completely new set of capabilities. Realizing CGTs typically involve time-dependent process steps and limited shelf-life, it is critical to have high quality raw materials and consumables readily available, have a robust process control process, and a process execution framework for successful production and treatment delivery to patients in a timely manner.
- Management and storage of raw material and consumables – The inability to manage key incoming raw material inventories (e.g. viral vector, media, buffer, and consumables), including limited capacity to store materials can cause interruptions in development and supply chains. To meet growing demand needs without disruptions, it is important to have a predictable availability of raw materials and consumables. Obtaining key knowledge during the development phase, with a focus on material robustness will allow for an adaptable framework to grow.
- Incoming raw materials testing time constraints – Considering the large number of quality control (QC) samples and complex assays to be performed per batch with limited time for process development, testing times creates a strain on QC capacity when production volumes increase. Any complications experienced during these QC processes can create significant delays and uncertainty in overall batch cycle time and increase costs, while causing knock-on effects in both manufacturing and supply chain. Awareness of this tight integration and options to manage it during growth is crucial. The concept of improved efficiencies and investment required in QC during ramp-up needs to be considered.
- Process execution for treatment delivery – Matching anticipated clinical and commercial demand and planning for the appropriate supply of the product is challenging when both raw material supply as well manufacturing capacity is constrained. Companies require a cross-functionally aligned product supply and delivery processes that are predictable as well as agile to accommodate inevitable process exceptions.
- Material Sourcing and Quality Management: The current focus of manufacturing of cell therapies is on individualized treatments, which brings the challenge of patient-specific variability. It is necessary to remove any other supply chain variabilities to minimize the risk profile during planned growth. Investment in quality during procurement and sourcing of raw materials, and partnerships with contract manufacturing organizations (CMOs), is necessary to ramp up these therapies to volume.
- High Demand/Limited Selection of CMOs in the CGT industry – Due to the growing demand for these products, capacity continues to be a challenge across the industry. The limited selection and capacity from CMOs and suppliers creates production bottlenecks and delays in the pipeline, from development through commercialization. The shortage of CMOs with relevant capacity and staff expertise has forced a growing number of companies to adapt a hybrid approach; or when the CMO option is simply not practical, to fully manufacture internally. Other companies are booking blocks of CMO time and capacity planning up to years in the future. It is key to develop a manufacturing and sourcing strategy early on by ensuring adequate capacity and resources based on the long-term business plan.
- Quantity and Quality of Raw Materials – Critical process components (e.g., viral vectors), where the quantity and quality of the component has significant impact on the final treatment release, must be managed under appropriate quality controls. These must be scalable and able to manage the capacity growth as QC assays mature and account for process variability. Sourcing key raw materials, such as viral vectors, have become a challenge due to limited selection of vendors and growing demand; companies have therefore opted to internalize their viral vector production to resolve this issue.
- High Cost of Goods Sold (COGS) – The high cost of producing new cell and gene therapies is one of the largest issues facing the industry, driving supply chain strategies and reputation. At launch, the price of Kymriah and Yescarta were $475,000 and $373,000 respectively.3 The COGS of these products can be as high as 30 – 40 percent of the product price. Primary drivers for the high cost are labor and direct materials. Expectations are that advances in manufacturing innovation will drive costs down; however, these advances must be considered earlier on. Once a therapy is approved, it is difficult and complex to make manufacturing changes after submission. Growth and process improvement strategies must be worked out thoroughly prior to submission.
2. Process Variability
In autologous cell therapies, the main challenge is managing variability when volume increases. For example, the manufacturing process is for one patient, one batch. This approach limits the traditional value typically gained by increasing batch size, which focuses on reducing overhead replication of operations (e.g., batch management processes, media preparation, sampling and labeling). Based on the complex nature of human cells and their sensitivity to environmental conditions outside the body, the overall yield of the final cell product can be impacted by many process conditions such as temperature, culture medium changes and even gas exchange. Multiple additional factors can also introduce variability to the process, such as:
- Material Variability: As the starting material for autologous cell therapies is derived from patients themselves, there is a wide range of viable cell count variability from person-to-person, and potential for unknown pre-existing conditions. The attributes and properties of the starting material makes it difficult to enable the manufacturing process to consistently deliver within a defined range of release attributes. The variability also leads to difficulty in predicting the viral vector forecasts to make patient doses.
- Low Process Efficacy: Cellular immunotherapies require careful orchestration of three main manufacturing components (plasmid DNA, viral vectors and cells). Large-scale production and long-term storage of viral vectors is not efficient in most cases, resulting in lower process yields, moderate purity and shorter shelf-life compared to recombinant protein therapeutics. Currently, more studies are needed around viral vector product quality impact and shelf-life.
- Talent and Expertise Scarcity: CGT manufacturing is a rapidly growing sector and remains a complex business that requires specific skills and capabilities in short supply. The technical complexity of CGT, the need for speed and the differences between development and commercial manufacturing require new specialized capabilities to drive the “right first time” process development that can be scaled. Creating an institutional knowledge is an elusive goal as expertise is worker specific and this individualized setup significantly contributes to the process variability. As these resources are in high demand, there is an inherent competitive aspect to retaining in-house trained expertise, which will be reflected in increasing costs. This must be considered with capacity increase. As the industry matures, companies will need to develop closed, efficient, and automated manufacturing operations.
3. Scalable Operations
- Ramp up & Capacity Growth: Allogenic cell therapies lend themselves to a scale-up manufacturing strategy, similar to monoclonal antibody (mAb) manufacturing. However – unlike mAb production—where the goal is to produce higher titers of cells containing the antibody—in cell-therapy, the cell itself is the therapeutic agent. The cells must keep their integrity and remain functional, two aspects that are challenged when the cell density in the manufacturing environment increases. Autologous cell therapies, in comparison, do not lend themselves to a scale-up manufacturing strategy because each treatment is patient specific. The entire manufacturing chain, from patient back to patient, must retain sterility and traceability. Thus, autologous cell therapies require a decentralized scaled-out manufacturing process—to produce a single batch of therapy for each patient. This poses a cost challenge which makes economies of scale difficult to achieve. Lastly, to move adeno-associated virus (AAV) gene therapy manufacturing process from small batch to a larger scale, scientists need to find new ways to efficiently culture cells in large containers. Typically, the cells grow on a surface in a plastic flask or bottle. However, to scale it up to generate sufficient material, it is best to have the cells in suspension. There is a need to find optimal ways to grow the cells to very high densities in suspension cultures while maintaining the specific productivity of the cells.
- Equipment and System Integration: Unlike traditional therapies, where manufacturing process variables have limited impact on patient outcomes, in CGT, patients are the bioreactors and there is a need to understand which manufacturing variable is most sensitive to the outcomes. The analysis requires access to both manufacturing and clinical outcomes data. Current scale-limiting, laboratory-based, single, serial manufacturing processes in a manufacturing clean room include equipment organized serially to complete a linear process. This linear system is often disconnected and limits equipment variety and introduces bottlenecks in the critical path. The process controls impede data visibility, increasing the time required for data gathering, reporting and analyses of process performance.
Removing Roadblocks – giving a jab of data in operations
Attempting to resolve these challenges post-launch is not efficient and would require a significant shift from current approaches. It is best to address these foundational challenges up-front by considering a paradigm shift from the traditional pharma “one-drug-fits-all” mindset to a new “personalized and agile view expecting change.” To embrace this shift, emerging cell therapy companies can adopt a three pronged approach to design and implement a new foundational operating model. The model is designed to implement a data-driven culture from early development phases, and use it to determine robust risk-based manufacturing and supply chain strategies to ultimately optimize commercialization (Figure 2).
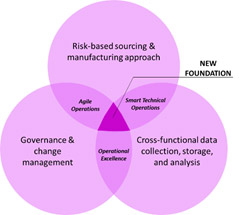
Figure 2. New Foundational Operating Model Elements
Based on an understanding of the current challenges in the industry, companies can evaluate key factors/considerations throughout the development cycle of new CGT assets to proactively mitigate risks and prevent anticipated challenges.
The new foundational operating model can enable forward thinking biopharma companies to become data-driven, agile and risk-based decision makers during the development phase. A strategic approach based on utilizing data to mitigate risks can enable smooth quality-related write-offs, save costs and ultimately reduce time-to-market. In Figure 3, we have listed key tactics, by each development phase, to address the challenges we previously identified in this article.

Conclusion
The predominant mode of operation for the manufacture of CGTs is batch-to-batch rather than continuous processing, requiring the industry to unlearn old ways and learn new ways of working. Currently, the market is not mature in terms of pivoting towards cell and gene therapies as a separate platform with more stringent needs than traditional biologics manufacturing. Due to the complex and living nature of the materials used in the process, and the smaller data sets to drive innovation, it will take time to develop standardization in the manufacturing process before ramping-up activities commence.
References
- Approved Cellular and Gene Therapy Products
Center for Biologics Evaluation and Research
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products - Roots Analysis: Cell Therapy Manufacturing Market (2nd Edition), 2018-2030
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing-market-2nd-edition-2018-2030/209.html - Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy, Hernandez et al.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6145722/
