Cost-Effective LV Production With The Next Generation Of Transfection Reagent

FectoVIR®-LV
Lentiviral vectors are recognized to be the carrier of choice for allogenic or autologous cell therapies (such as CAR-T) because of its capacity to permanently integrate viral genome into host cell DNA. To produce those vectors, cell therapy producers generally use a transient transfection system that is scaled-up during process development phases. FectoVIR®-LV is the next generation of transfection reagent, free of animal component, designed to improve LV productivity in HEK-293 cell systems. FectoVIR-LV is made for large scale manufacturing with reduction of the complexation volume and increased complex stability. The benefits that FectoVIR-LV bring allow to increase number of doses produced per batch to treat more patient, while decreasing manufacturing costs.
| Product information |
Ready to use, synthetic and free of any animal-origin component |
|---|---|
| Applications |
Lentiviral vectors (LV) manufacturing using HEK-293 derivative cellscultivated in suspension |
| Number of transfections |
100 mL of FectoVIR®-LV transfection reagent is sufficient to transfect on average 50 L of cell culture (using standard conditions) |
| Storage condition |
Store FectoVIR®-LV at 5 °C ± 3°C. |
- Productivity: Reach highest Lentiviral vectors titers in suspension systems
- Cost-effectiveness: Reduce cost per batch with high titers and low DNA consumption
- Scalability: Produce at large scale with low complexation volume and long complex stability
- Market faster: Rely on DOE service to optimize your process in a record time
Productivity
The production of lentiviral vectors for cell and gene therapy is performed using 3rd generation plasmid systems in HEK-293 and derivative cell systems. Transfection reagents are considered as a critical raw material because of its major impact on productivity. Choosing the transfection reagent based on the best titers is a must for process economics and intensification.
FectoVIR®-LV increases up to 3-fold functional titers in comparison to other commercially available lentivirus-specific transfection reagents (Fig.1)
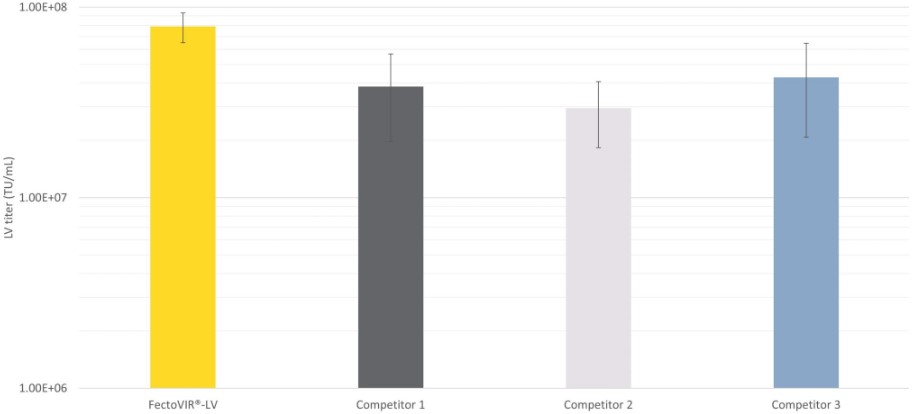
Fig 1. FectoVIR®-LV demonstrates a high productivity for lentiviral vector manufacturing compared to competitors in the field. Lentiviral vectors were produced using FectoVIR®-LV or 3 other competitors and a 4-plasmid system in 125 mL flask with HEK-293T cell cultivated in suspension. The transfection mix was incubated during 15, 30 or 45 min, using the recommended conditions (ratio DNA:transfection reagent of 1:1, 1 µg of DNA per million cells, 5% complexation volume) and LV were harvested 72h post-transfection. Functional titers were measured using an infectivity test.
Cost-effectiveness
Improving titers is one of the key parameters to increase number of doses per batch and reduce cost per dose. When combined with reduced .consumption of additional critical raw material such as DNA quantity, overall manufacturing costs can be further reduced. FectoVIR®-LV both allows gain in productivity while reducing by 2-fold DNA consumption (Fig.2). To support our customers, Polyplus offers a comprehensive DOE service to optimize key parameters of transfection step to reach higher titers.
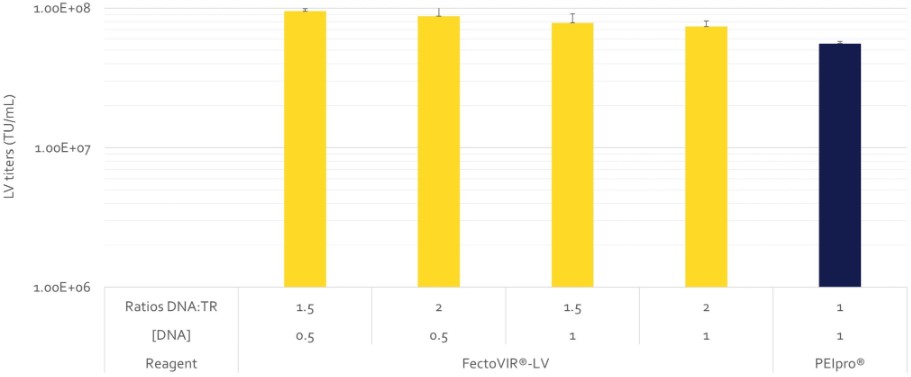
Fig 2. FectoVIR®-LV transfection protocol uses less DNA quantity and optimizes titers to improve process economics. Lentiviral vectors were produced using FectoVIR®-LV or PEIpro® and a 4-plasmid system in 125 mL flask with HEK-293T cell cultivated in suspension. The transfection mix was incubated during 30 min, using different ratio DNA:transfection reagent (from 1.5 to 2), DNA quantity (from 0.5 to 1 µg/10E6 cells) using FectoVIR®-LV and using recommended transfection protocol with PEIpro®. LV were harvested 72h post-transfection. Functional titers were measured using an infectivity test.
Scalability
Transient transfection at large scale comes with challenges, of which transfer of transfection mix within an optimal timeframe. This step can be a limit if the transfection reagent is not adapted for large scale manufacturing. FectoVIR®-LV has been designed to impact both complex stability and complex volume. Increasing the complex stability and reducing the volume of complexation allows sufficient time to transfer the mix into large bioreactors (>200 L). FectoVIR®-LV has proven its efficiency using the recommended 5% complexation volume and 30 min of complexation volume (Fig 3.), which can be even further optimized to fit your process. Polyplus recommends performing a DOE study adapted to your system.
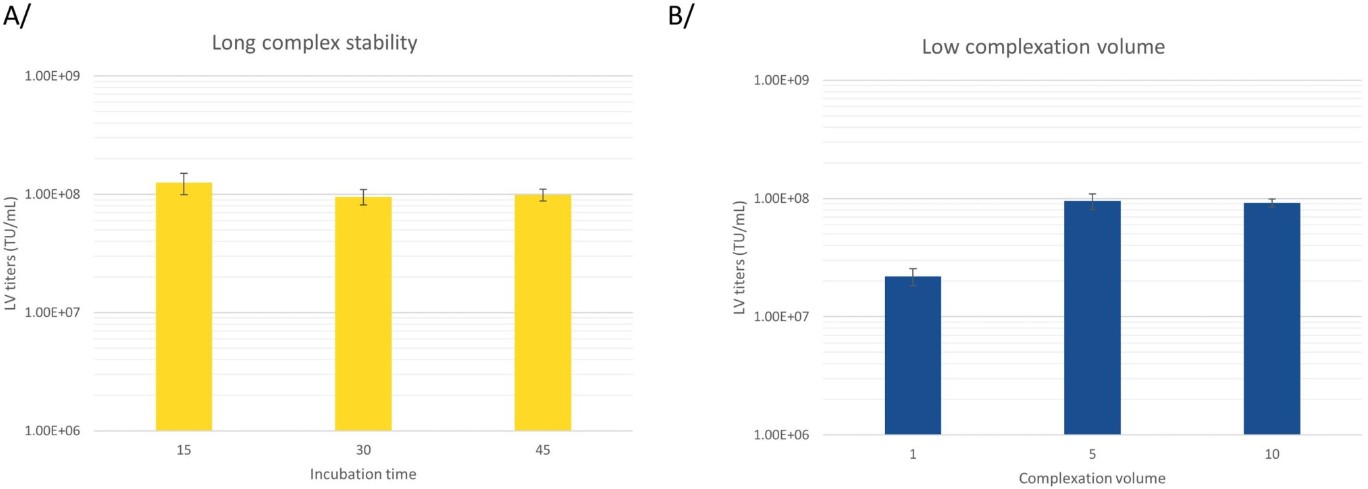
Fig 3. FectoVIR®-LV is highly scalable with recommended conditions allowing to transfect large bioreactors. Lentiviral vectors were produced using FectoVIR®-LV and a 4-plasmid system in 125 mL flask with HEK-293T cell cultivated in suspension. A/ The transfection mix was incubated during 15, 30 or 45 min, using the recommended conditions (ratio DNA:transfection reagent of 1:1, 1 µg of DNA per million cells, 5% complexation volume) and rLV were harvested 72h post-transfection. Functional titers were measured using an infectivity test. B/ The transfection mix were complexed using in 1, 5 or 10% of the total final volume (30 mL), using the recommended conditions (ratio DNA: transfection reagent of 1:1, 1 µg of DNA per million cells, 30 min incubation time) and LV were harvested 72h post-transfection. Functional titers were measured using an infectivity test.
| Part Number | Designation |
|---|---|
| 101000187 | FectoVIR®-LV 1 mL |
| 101000188 | FectoVIR®-LV 10 mL |
| 101000189 | FectoVIR®-LV 100 mL |
