Combatting Unproven Cell And Gene Therapies Through Regulation And Education
By Suzanne Elvidge, Contributing Writer
Follow Me On Twitter @suzannewriter
Cell and gene therapies (CGTs), once a dream of science fiction, are now a reality of modern medicine across the developed world. Growing numbers of proven CGTs have gained marketing approval and are available to provide treatments for patients, mostly in life-threatening and rare diseases.

With this comes a challenge. Alongside the rise in proven, evidence-based, and authorized treatments is a parallel increase in unproven and unlicensed cell-based therapies and interventions.

COMBATTING UNPROVEN THERAPIES THROUGH EDUCATION
CGTs that have not gone through thorough preclinical and clinical testing and approval may simply be ineffective and raise unrealistic hopes for vulnerable patients. Alternatively, they may actually be harmful, causing side effects or permanent damage.
To try to combat unproven treatments, Laertis Ikonomou, Ph.D., of the Pulmonary Center and the Center for Regenerative Medicine, Boston University, and Natividad Cuende of the Andalusian Initiative for Advanced Therapies and Andalusian Transplant Coordination, Spain, came up with the idea of the ISCT annual report. It aims to create a central resource that will help health-care professionals and researchers identify those CGTs that are proven safe and effective through rigorous scientific and regulatory assessment.
ISCT also wants to work with patient associations, drug companies, and HCPs to teach patients about the dangers of unapproved therapies. Good information needs to be available where patients go to find it, and the internet and social media are often first stops. There are patient groups using Facebook that can provide good information, but there are also misleading websites that drive patients to unproven therapies.
“Patients need to be educated and able to ask the right questions. They must make their own choices and provide informed consent, but this requires the right information,” said Dominici.
The ISCT also wants to provide a dedicated ISCT website where patients can find information and make educated decisions about therapies that they are offered.
“Patients need to have advice and information about safe and effective treatments available in their country, including whether a medicine is available as part of a clinical trial. Our aim is to provide information and equip patients to make decisions,” said Cuende.
MAKING THE MOST OF THE OPPORTUNITIES FOR PROVEN THERAPIES
Companies need to first demonstrate the science, then the manufacturing, and finally the effectiveness of a drug. “My advice for companies working in this area is to understand the science behind the product, know how it works, have a robust manufacturing program, and start by focusing on rare diseases. Novartis’ Kymriah is a good example. It used good science, it’s effective, there is a manufacturing process, and it targets B-cell acute lymphoblastic leukemia, a rare disease,” said Dominici.
Manufacturing is always going to be a challenge for CGTs, especially as therapeutic targets move from rare and ultrarare diseases to more common conditions. Some CDMOs are scaling up to meet the coming need, but despite this, finding capacity can be a challenge, and many companies are seeking to set up or expand their own in-house production. Examples include Orchard Therapeutics, which is building a gene therapy manufacturing site in Fremont, CA, and Novartis, which has made an offer to acquire CellforCure, a French contract manufacturer that already produces Kymriah. “How we produce CGTs has been improving through an industrial revolution in manufacturing,” said Dominici.
Proving effectiveness is an important step, according to Miguel Forte, CEO of Zelluna Immunotherapy, a biotech company specializing in T-cell receptor (TCR) immunotherapies for solid tumors. “Companies need to focus on proven therapies, providing value that’s driven by evidence,” said Forte. “We have seen documented benefits, but our products still need preclinical and clinical testing before regulatory approval and the market. The right kind of documentation adds value.”
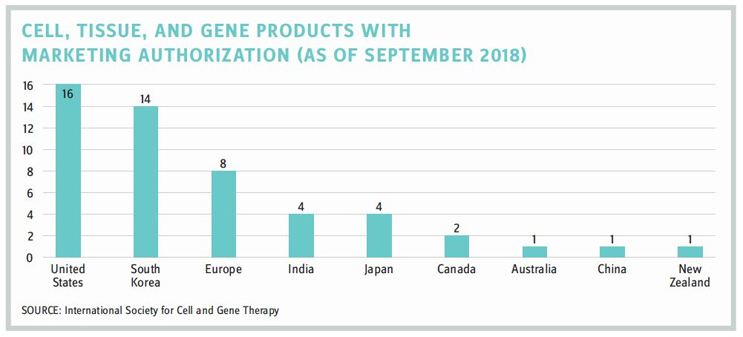
Companies need to decide which market or territory they will target. On the surface, the U.S. has the largest number of approved CGTs on the market, at 16, set against South Korea’s 14 and Europe’s eight. However, as Cuende explained, half of the CGTs in the United States are umbilical cord products, which are regarded by Europe as transplants rather than medicinal products. If these are taken away, Europe and South Korea are the locations with the most approved products. There is a balance though — while South Korea is the place where these therapies are the most accepted to date, the U.S. and Europe are by far the largest markets.
BUILDING AND MOVING FORWARD THROUGH REGULATION
Development of CGTs has sped up over the last two years, capitalizing on the growth in academic research and the streamlining of the regulatory processes. This has meant a steep learning curve for the regulatory authorities, especially with cell-based therapies that require regulators to deal with a treatment that has both a donor and a patient. As such, ISCT has been working with the FDA to develop guidance documents.
According to Dominici, the regulatory authorities have been proactive, and regulation and development are becoming well-aligned. He suggests the next step could include an accelerated path of development for orphan and rare drugs.
Of course, there always will be a tension between speed and safety in drug development, and it’s no different for CGTs. “In the last two or three years, there has been a sensitivity from the regulatory authorities to accelerate, as most CGTs are targeting areas of unmet need,” added Cuende. “This includes the FDA’s regenerative medicine advanced therapy [RMAT] designation, the European Medicines Agency’s PRIME: priority medicines, and Japan’s conditional approval pathway for regenerative medicine.” The challenge for the regulatory authorities is that there are few precedents, as there is only a handful of global CGTs approved so far.
The ISCT’s report will be a regularly updated and republished annually. It is accessible at www.celltherapysociety.org and www.isct-unprovencellulartherapies.org.
