Cell Expansion Bioreactor System: LiCellGrow

Visualize continuous metabolic changes in cells in real-time and adjust cell culture conditions automatically for enhanced cell quality.
Ensuring cell quality and stability is vitally important in the manufacturing of cell and gene therapy products such as cells for CAR-T therapy. However, manufacturing with cells as raw materials can be affected by factors such as variations in the manufacturing process and in the characteristics of the cells, which makes product quality inconsistent. Understanding the state of cells throughout the manufacturing process is an essential factor for improving the quality of cell and gene therapy products.
PHCbi is currently developing an innovative cell expansion system, LiCellGrow™ with the goal of addressing these challenges. Our unique In-Line Sensor is being designed to monitor cell culture in real time, which would allow automatic control of the culture to match the metabolic state of the cells while maintaining sterile conditions. This is intended to enable the state of the cells to be quantified, greatly improving the efficiency and consistency of the manufacturing process.
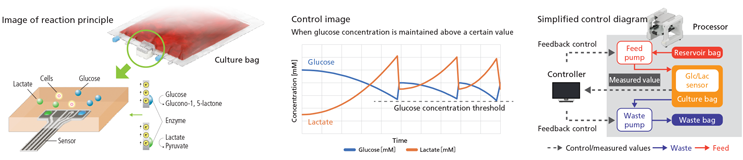
Continuous measurement by the In-Line Sensor will allow visualization of changes in glucose and lactate concentrations. Automatic culture medium replacement will ensure an optimal culture environment at all times.
Glycolysis is one of the major energy metabolism pathways in cells. Since this process takes in glucose and produces lactate, changes in these substances are considered to be a useful indicator of the cellular state. The LiCellGrow cell expansion system is intended to not only conventionally detect pH/DO in the medium, but also measure the concentrations of glucose and lactate in real time. It is being designed to be capable of automatically replacing the culture medium based on the measured glucose and lactate values.
The dedicated culture bag will be fitted with the PHCbi In-Line Sensor, with the intent to provide real-time measurements of glucose and lactate as continuous line data. The measured values will be converted to concentrations to visualize cell status, and this information is intended to be used to automatically replenish the medium and drain waste. This will ensure that glucose and lactate concentrations in the medium are controlled and that cells are always cultured in their most suitable environment.
Closed system culture with single-use culture bags
Highly reproducible culture with guaranteed sterility
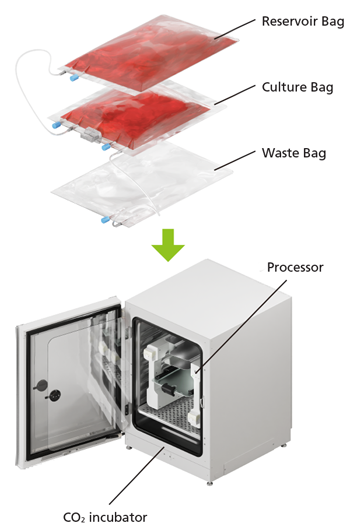 The gas-permeable dedicated culture bags and the In-Line Sensor is being designed for single use only, and gamma-irradiated before supply. The system will simply need to be fitted to rapidly start culture and maintain a sterile environment. The processor will be installed inside the customer's CO2 incubator, which controls temperature and CO2 to allow culture in a highly reliable and reproducible environment. With measurement automated by In-Line monitoring, this system is intended to eliminate the need for sampling, greatly reducing the risk of contamination.
The gas-permeable dedicated culture bags and the In-Line Sensor is being designed for single use only, and gamma-irradiated before supply. The system will simply need to be fitted to rapidly start culture and maintain a sterile environment. The processor will be installed inside the customer's CO2 incubator, which controls temperature and CO2 to allow culture in a highly reliable and reproducible environment. With measurement automated by In-Line monitoring, this system is intended to eliminate the need for sampling, greatly reducing the risk of contamination.
Monitoring of the cell culture process for cell management with a high level of quality control. Contributing to process development based on the QbD concept.
Quality by Design (QbD) emphasizes consideration of product quality right from the design stage. Visualizing the concentrations of glucose and lactate in cell culture is essential for ensuring cell product quality. It enables the condition of cells in culture to be directly determined metabolically, which is difficult with conventional pH/DO monitoring, leading to optimization of the cell culture process. Using this functionality to create the desired culture environment will help in the development of processes based on the QbD philosophy.
