Application Of CRISPR Technology In iPSC Gene Correction
By Jack (Jie) Huang, M.D., Ph.D.
The rise of induced pluripotent stem cell (iPSC) technology has opened up a new path for regenerative medicine and the treatment of genetic diseases. By reprogramming somatic cells into pluripotent stem cells, researchers can obtain an unlimited source of cell resources with the patient’s genetic background and induce them to differentiate into various functional cell types for disease modeling, drug screening, and cell replacement therapy.1 At the same time, the advent of the CRISPR-Cas9 system provides an efficient and programmable gene editing tool, making it possible to accurately repair specific sites.2
The combination of iPSC and CRISPR technology not only breaks through the limitations of donor shortages and immune rejection in traditional cell therapy, but also provides a realistic and feasible strategy for personalized treatment of single-gene genetic diseases.3 In the patient’s own iPSCs, the pathogenic mutation is repaired by CRISPR, and then the cells are directed to be induced into targeted cells and then reinfused into the body, which can achieve a “cause correction plus immune compatibility” treatment model.4 In addition, this platform also lays the foundation for creating disease models in isogenic backgrounds, studying the functions of genetic variants, and screening personalized drugs.5 With the continuous advancement of editing efficiency, differentiation control, and safety assessment technologies, the CRISPR-iPSC combined system is increasingly becoming a core tool for precision medicine.
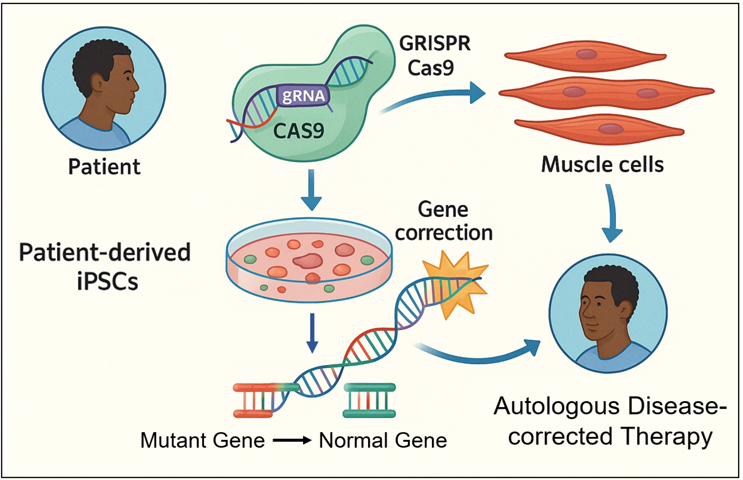
Fig 1. CRISPR Technology in iPSC Gene Correction. This figure illustrates the application of CRISPR-Cas9 technology for gene correction in patient-derived induced pluripotent stem cells (iPSCs). Cells obtained from the patient are reprogrammed into iPSCs carrying the patient's genetic mutation. Targeted gene correction is performed using the CRISPR-Cas9 system, replacing the mutant gene with a normal allele. Then, the corrected iPSCs can be differentiated into functional cell types, such as muscle cells, and potentially reintroduced into the same patient as an autologous therapy. This personalized approach enables the development of curative therapies for monogenic diseases while minimizing the risk of immune rejection.
Overview Of The CRISPR Gene Editing Mechanism
The CRISPR (clustered regularly interspaced short palindromic repeats) gene editing system is derived from the acquired immune mechanism of bacteria. Its core is the synergistic effect of Cas nuclease and specific guide RNA (sgRNA).6 The most commonly used Cas9 protein can cause double-strand breaks (DSBs) at the target DNA site, and then rely on the cell’s own non-homologous end joining (NHEJ) or homologous recombination (HDR) mechanism to complete gene knockout or repair.7 However, DSBs may cause chromosomal rearrangements or off-target mutations, which require careful evaluation in iPSCs.8 To improve safety and accuracy, researchers have developed base editing and prime editing systems that do not rely on DSBs.
Base editing achieves single-base conversions such as C→T or A→G by fusing deaminase and Cas9 nickase, avoiding the risk of causing breaks and is suitable for correcting point mutations.9 Guided editing further expands the editing range and, using reverse transcriptase and pegRNA templates, can achieve insertions, deletions, and multiple types of base substitutions.10 The application of the CRISPR system in iPSCs has the advantages of high controllability, clonal screening, and long-term amplification, making it easy to complete high-precision repairs and verify functions in vitro.11 However, there are also challenges, such as chromosomal instability, off-target accumulation, and low HDR efficiency, which need to be controlled with high-throughput sequencing and clonal screening mechanisms.12
Typical Cases Of Single-Gene Disease Correction
Using CRISPR to precisely repair pathogenic gene mutations in iPSCs is currently one of the most promising directions for treating single-gene genetic diseases. The following takes three representative genetic diseases as examples to introduce their mutation characteristics, editing strategies, differentiation effects, and functional verification.
1. Duchenne muscular dystrophy (DMD)
DMD is an X-linked recessive genetic disease, usually caused by large deletions or missense mutations within the DMD gene, resulting in loss of dystrophin function.13 Traditional treatments can only alleviate symptoms, while CRISPR technology makes radical treatment possible. In iPSCs derived from DMD patients, researchers often use exon skipping strategies to remove introns or abnormal exons near the gene mutation through CRISPR-Cas9, realign the reading frame, and thus restore downstream protein expression .14 For example, deletion of exons 45 – 55 can cover the common gene mutation region in most patients.15 After gene-edited iPSCs were successfully differentiated into skeletal muscle progenitor cells, they were able to express truncated but functional dystrophin protein in vitro and exhibit contractile function, verifying its biological repair effect.16
2. Sickle cell disease and β-thalassemia (SCD/β-thalassemia)
Both of these hemoglobinopathies are caused by HBB gene mutations. The former is a Glu6Val point gene mutation, while the latter is mostly caused by promoter mutations, missense mutations or splice site mutations, resulting in reduced or absent β-chain synthesis.17 For HBB mutations, CRISPR-Cas9 can be combined with a donor template (ssODN) to achieve precise base repair through HDR mechanism.18
In iPSCs derived from SCD patients, after the Glu6Val mutation is successfully repaired, erythroid progenitor cells can be differentiated and synthesize normal adult hemoglobin (HbA) in vitro, while reducing the production of pathological HbS.19 Functional testing showed that these cells no longer develop typical sickle deformation under hypoxic conditions, suggesting that mutation correction has functional efficacy.20 Another strategy is to activate the fetal hemoglobin genes HBG1/2 in iPSCs to functionally replace the damaged HBB. Researchers achieved this by interfering with the BCL11A enhancer region using CRISPR, and the related cells have verified their therapeutic potential in animal models.21
3. Cystic fibrosis (CF)
CF is caused by mutations in the CFTR gene, the most common of which is the ΔF508 deletion mutation, which leads to CFTR protein misfolding, localization disorder and loss of Cl⁻ channel function.22 Using CRISPR-Cas9 combined with HDR strategy to repair this mutation in iPSCs from CF patients can restore the expression of CFTR protein on the cell membrane. The researchers induced the repaired iPSCs into airway epithelial cells and tested their Cl⁻ channel function. They found that they showed CFTR activity similar to that of normal cells in the electrophysiological Ussing chamber test and responded normally to small molecule activators .23 In addition, the restoration of mucus secretion function was also observed in airway organoids constructed based on iPSCs, providing theoretical support for subsequent clinical transplantation .24
Safety And Quality Control Strategies
In the CRISPR-mediated iPSC editing process, genome integrity and editing accuracy are prerequisites for clinical application. To ensure accurate editing, a cloning strategy is often used, that is, the edited single-cell clones are amplified, screened, and verified to exclude cells that are not fully repaired, have low editing efficiency, or carry unexpected mutations.25 Off-target effect detection is the core step in evaluating editing safety. The current mainstream methods include high-throughput technologies such as GUIDE-seq, Digenome-seq, and CIRCLE-seq, which can systematically evaluate gRNA-induced non-specific cutting sites.26 In addition, the use of high-fidelity Cas9 mutants (such as eSpCas9 and HiFi-Cas9) can further reduce the risk of off-target effects.27
Similarly, genome stability analysis cannot be ignored, because long-term cultured iPSCs may have chromosomal aberrations or structural rearrangements. Through karyotype analysis, SNP chips, whole genome sequencing, etc., the number and structural changes of cloned chromosomes can be effectively monitored.28
In terms of preclinical quality standards, regulatory agencies (such as the FDA and EMA) require that cell products have clear parameters for identity, purity, potency, and safety.29 Therefore, an editing process that complies with GMP standards, clear release standards, and traceable quality records are the basic guarantees for CRISPR-iPSC products to enter clinical trials.30
Preclinical Research And Translational Potential
Multiple animal experiments have verified that CRISPR-repaired iPSC-derived cells have functional repair capabilities and preliminary safety.
For example, in a DMD mouse model, CRISPR-repaired iPSCs differentiated into muscle cells and then transplanted can significantly improve muscle contraction function and restore some dystrophin expression.31 In a study of β-thalassemia, repaired iPSC-HSC-like cells can sustainably express β-globin and reconstruct some hematopoietic function after being re-infused into immunodeficient mice.32
The key advantage of autologous iPSC therapy is immune compatibility to avoid rejection. However, its clinical translation still faces multiple challenges, including: insufficient functional maturity of edited cells, the impact of the in vivo microenvironment on the integration of transplanted cells, and imperfect long-term safety assessment after transplantation.33 In addition, the long production cycle and high cost of autologous therapy also limit its large-scale application.34 In the future, optimizing the differentiation process, improving cell implantation efficiency, and establishing a long-term follow-up system will be the key path for CRISPR-iPSC personalized therapy to move from animal experiments to the clinic.35
Conclusion
The combination of CRISPR and iPSC technology provides a powerful tool for the precision treatment of genetic diseases. By efficiently repairing pathogenic gene mutations in patient-derived iPSCs and inducing their differentiation into functional cells for reinfusion, truly personalized cell therapy is possible. Looking ahead, this platform is gradually moving toward clinical translation and holds a promising future.
References:
- Jonas Cerneckis et al., Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduction and Targeted Therapy 2024, 9: 112. (https://doi.org/10.1038/s41392-024-01809-0)
- Darakhshan Javaid et al., CRISPR/Cas9 system: a reliable and facile genome editing tool in modern biology. Molecular Biology Reports 2022, 49: 12133-12150. (doi: 10.1007/s11033-022-07880-6)
- Avinash Singh et al., A high efficiency precision genome editing method with CRISPR in iPSCs. Scientific Reports 2024, 14: 9933. (https://doi.org/10.1038/s41598-024-60766-4)
- [4] Yoon-Young Jang and Zhaohui Ye, Gene correction in patient-specific iPSCs for therapy development and disease modeling. Human Genetics 2016, 135: 1041-1058. (doi: 10.1007/s00439-016-1691-5)
- Martina Celotti et al., Protocol to create isogenic disease models from adult stem cell-derived organoids using next-generation CRISPR tools. STAR Protocols 2024, 5: 103189. (https://doi.org/10.1016/j.xpro.2024.103189)
- Md Zohaib Ahmed et al., Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas advancement in molecular diagnostics and signal readout approaches. The Journal of Molecular Diagnostics 2021, 23: 1433-1442. (https://doi.org/10.1016/j.jmoldx.2021.07.025)
- Michael Kosicki et al., Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nature Biotechnology 2018, 36: 765-771. (https://doi.org/10.1038/nbt.4192)
- Chaoyou Xue and Eric Greene, DNA repair pathway choices in CRISPR-Cas9 mediated genome editing. Trends in Genetics 2022, 37: 639-656. (doi: 10.1016/j.tig.2021.02.008)
- Ariel Kantor et al., CRISPR-Cas9 DNA base-editing and prime-editing. International Journal of Molecular Sciences 2020, 21: 6240. (doi: 10.3390/ijms21176240)
- Youcai Xiong et al., EXPERT expands prime editing efficiency and range of large fragment edits. Nature Communications 2025, 16: 1592. (https://doi.org/10.1038/s41467-025-56734-9)
- Connor Wiegand and Ipsita Banerjee, Recent advances in the applications of iPSC technology. Current Opinion in Biotechnology 2019, 60: 250-258. (https://doi.org/10.1016/j.copbio.2019.05.011)
- Congting Guo et al., Off-target effects in CRISPR/Cas9 gene editing. Frontiers in Bioengineering and Biotechnology 2023, 11: 1143157. (doi: 10.3389/fbioe.2023.1143157)
- Addeli Angulski et al., Duchenne muscular dystrophy: disease mechanism and therapeutic strategies. Frontiers in Physiology 2023, 14: 1183101. (doi: 10.3389/fphys.2023.1183101)
- Yuto Kita et al., Dual CRISPR-Cas3 system for inducing multi-exon skipping in DMD patient-derived iPSCs. Stem Cell Reports 2023, 18: 1753-1765. (DOI: 10.1016/j.stemcr.2023.07.007)
- Javier Poyatos-Garcia et al., Deletion of exons 45 to 55 in the DMD gene: from the therapeutic perspective to the in vitro model. Skeletal Muscle 2024, 14: 21. (doi: 10.1186/s13395-024-00353-3)
- Federica Iberite et al., Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regenerative Medicine 2022, 7: 23. (https://doi.org/10.1038/s41536-022-00216-9)
- Tang-Her Jaing et al., Molecular genetics of β-thalassemia. Medicine 2021, 100: e27522. (doi: 10.1097/MD.0000000000027522)
- Hongyu Liao et al., CRISPR-Cas9-mediated homology-directed repair for precise gene editing. Molecular Therapy Nucleic Acids 2024, 35: 102344. (https://doi.org/10.1016/j.omtn.2024.102344)
- Xiaosong Huang et al., Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells 2015, 33: 1470-1479. (doi: 10.1002/stem.1969)
- Patricia Kavanagh et al., Sickle Cell Disease. JAMA 2022, 328: 57-68. (doi:10.1001/jama.2022.10233)
- Andres Lamsfus-Calle et al., Comparative targeting analysis of KLF1, BCL11A, and HBG1/2 in CD34+ HSPCs by CRISPR/Cas9 for the induction of fetal hemoglobin. Scientific Reports 2020, 10: 10133. (doi: 10.1038/s41598-020-66309-x)
- Carlos Farinha and Isabelle Callebaut, Molecular mechanisms of cystic fibrosis – how mutations lead to misfunction and guide therapy. Bioscience Reports 2022, 42: BSR20212006. (doi: 10.1042/BSR20212006)
- Hudson Harris and Javeed Kittur, Unlocking the potential of CRISPR-Cas9 for cystic fibrosis: A systematic literature review. Gene 2025, 942: 149257. (https://doi.org/10.1016/j.gene.2025.149257)
- Lin Lin et al., Human airway submucosal gland organoids to study respiratory inflammation and infection. Cell Stem Cell 2025, 32: 1170-1182. (DOI: 10.1016/j.stem.2025.05.013)
- Hyunsoo Jang et al., Chapter 10 - CRISPR/Cas9 technologies to manipulate human induced pluripotent stem cells. Methods in iPSC Technology 2021, 9: 249-287. (https://doi.org/10.1016/B978-0-323-85766-6.00012-7)
- Jinjing Li et al., Advances in detecting and reducing off-target effects generated by CRISPR-mediated genome editing. Journal of Genetics and Genomics 2019, 46: 513-521. (https://doi.org/10.1016/j.jgg.2019.11.002)
- Daisuke Matsumoto et al., SpCas9-HF1 enhances accuracy of cell cycle-dependent genome editing by increasing HDR efficiency, and by reducing off-target effects and indel rates. Molecular Therapy – Nucleic Acids 2024, 35: 102124. (doi: 10.1016/j.omtn.2024.102124)
- Stephen Attwood and Michael Edel, iPS-cell technology and the problem of genetic instability—can it ever be safe for clinical use? Journal of Clinical Medicine 2019, 8: 288. (doi: 10.3390/jcm8030288)
- Lisa Plitnick, Global regulatory guidelines for vaccines. Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics 2013, 28: 225-241. (doi: 10.1016/B978-0-12-394810-6.00009-5)
- FDA, Regulations: good clinical practice and clinical trials. (https://www.fda.gov/science-research/clinical-trials-and-human-subject-protection/regulations-good-clinical-practice-and-clinical-trials)
- Yue Jin et al., CRISPR/Cas9 technology in restoring dystrophin expression in iPSC-derived muscle progenitors. J Vis Exp 2019, 151: 10.3791/59432. (doi: 10.3791/59432)
- Yexing Xian et al., The safety and effectiveness of genetically corrected iPSCs derived from β-thalassaemia patients in nonmyeloablative β-thalassaemic mice. Stem Cell Research & Therapy 2020, 11: 288. (doi: 10.1186/s13287-020-01765-w)
- Mahsa Gheitasi et al., Generation of immune cells from induced pluripotent stem cells (iPSCs): Their potential for adoptive cell therapy. Human Immunology 2024, 85: 110836. (https://doi.org/10.1016/j.humimm.2024.110836)
- Natalie Francis et al., Development of an automated manufacturing process for large-scale production of autologous T cell therapies. Molecular Therapy-Methods & Clinical Development 2023, 31: 101114. (https://doi.org/10.1016/j.omtm.2023.101114)
- Mahima Choudhury et al., Advancing cell therapies with artificial intelligence and synthetic biology. Current Opinion in Biomedical Engineering 2025, 34: 100580. (https://doi.org/10.1016/j.cobme.2025.100580)
About The Author:
 Dr. Jack (Jie) Huang is currently the president at American Association for Scientist Entrepreneurship (AASE), the director at AASE Institute, the editor-in-chief of AASE Institute Journal (Science-to-Market Review), the vice president at American Botanical Drug Association (ABDA), the executive director of ABDA Journal (Botanical Drug), the visiting professor, chief scientist and founder/CEO at CSTEAM Biotechnology in Ohio. Dr. Huang is the author of chapters in two academic books, “Advanced Concepts and Strategies in Central Nervous System Tumors” and “Challenge of Glioblastoma - From Pathology to Survival,” and five medical science popularization books. His research and development interests have been focused on biological models, biochips, and CAR-T cell therapy.
Dr. Jack (Jie) Huang is currently the president at American Association for Scientist Entrepreneurship (AASE), the director at AASE Institute, the editor-in-chief of AASE Institute Journal (Science-to-Market Review), the vice president at American Botanical Drug Association (ABDA), the executive director of ABDA Journal (Botanical Drug), the visiting professor, chief scientist and founder/CEO at CSTEAM Biotechnology in Ohio. Dr. Huang is the author of chapters in two academic books, “Advanced Concepts and Strategies in Central Nervous System Tumors” and “Challenge of Glioblastoma - From Pathology to Survival,” and five medical science popularization books. His research and development interests have been focused on biological models, biochips, and CAR-T cell therapy.
