Advanced Therapies In The UK: A Recipe For Success?
By Ian Hollingsworth, associate director of product development, Boyds
Ian Hollingsworth, associate director of product development at Boyds, the global drug development consultancy, discusses the current landscape for advanced medicinal therapies (ATMPs), explains the benefits and challenges of developing and adopting them, and outlines best practice resources and schemes developed in the UK that are supporting and accelerating the delivery of new, cutting-edge medicines to patients.
Introduction
Once again, 2021 managed to break records in the area of advanced medicinal therapeutic products (ATMPs). The global financing of companies developing advanced therapies increased significantly, by 16% on the 2020 figure with $23.1 billion raised. In the UK alone, the number of ATMP clinical trials has again increased, up 9% from 2020 to a figure of 168 which accounts for 12% of trials being run globally1. Both metrics continue a year-on-year increase demonstrating the expectation and confidence in this growing field. We now have evidence that genetically modified cell therapies, such as CAR-T (Chimeric Antigen Receptor – T cells), can have a significant and long-lasting beneficial effect2 that one would expect to increase the confidence of regulators and investors in this field.
Advanced therapies potentially offer considerable clinical benefits to patients, altering the course of diseases which have failed to be adequately treated with existing methods. However, there remain many challenges to the wide-scale adoption of ATMPs, even after they have been approved by the relevant regulatory agencies and health authorities in a particular country.
In the UK, the government has recognised the potential of advanced therapies, both to treat patients but also as an opportunity to excel in manufacturing these high value medicines and has instigated programmes to maximise these opportunities.
The current ATMP landscape
The advanced therapy ecosystem is still relatively small in comparison to the global biotech arena. Annual reports produced by groups such as the Alliance for Regenerative Medicine (ARM)3 and the Cell and Gene Therapy Catapult4 globally5 and across the UK1 respectively, show a steady increase over the last few years in the number of companies globally working to develop new advanced therapies, with currently 1308 developers worldwide identified, representing a 19% increase over the number from 2020 (Figure 1).
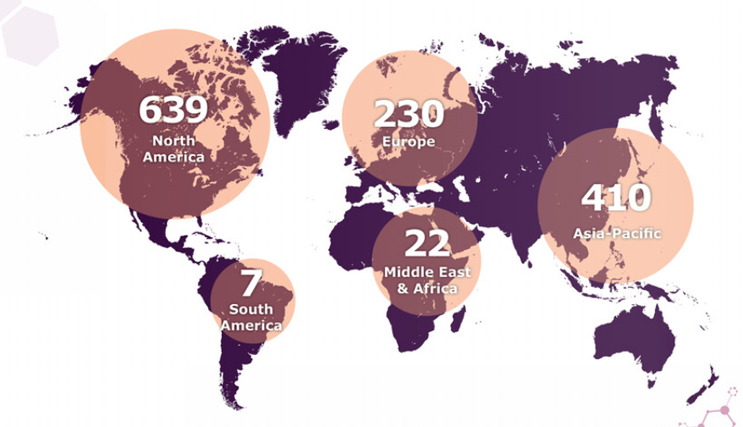
Figure 1: Location of ATMP developers6
Over the past five years, clinical trials of ATMPs have increased significantly, with some 2406 trials being run globally in 2021, despite the Covid pandemic having had a significant impact on business operations. This represents almost a doubling from the 1220 trials in 2020, and a 2.5-fold increase on the 2019 figure, demonstrating that there was a considerable upward trajectory irrespective of the pandemic.
In the UK, there has been a steady increase in trial numbers as shown in Figure 2, with 168 trials running as of the end of 2021.1
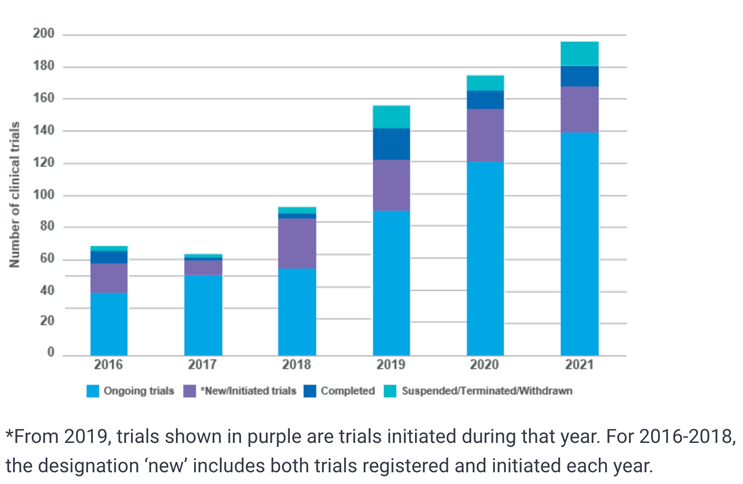
Figure 2: Number of ongoing, initiated, completed and closed trials 2016-20211
The majority of ongoing and new clinical trials are currently recruiting new patients, allowing wider access to these ground-breaking treatments. Further analysis carried out by the Cell and Gene Therapy Catapult (Figure 3) shows that the majority of trials are in clinical Phase I/II. This is encouraging as, during the natural progression of clinical trials, more patients will be recruited and the experience of setting up and running these trials will be shared across multiple centres, spreading the expertise into less experienced hospitals. This expansion across multiple centres helps to disseminate the knowledge and experience of how to conduct clinical studies of ATMPs and allay some natural concerns from clinical sites/investigators about working with ATMPs and what infrastructure is required, when it is new.
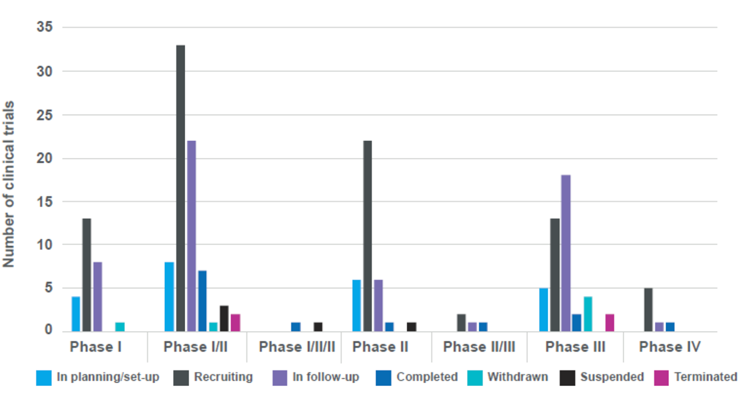
Figure 3: Distribution of UK clinical trials of ATMPs by clinical phase and status1
2021 proved to be a watershed year for cell and gene therapies, with six new therapies approved around the world, including new therapies for cancer, severe burns, and neurological disorders. The outlook for 2022 and beyond is equally exciting, if not more so, with a significant number of expected regulatory decisions to be announced as shown in Figure 4. It is widely quoted that the regulatory agencies - FDA, EMA and MHRA - all expect to be reviewing five to ten advanced therapy Marketing Applications a year from 2025.
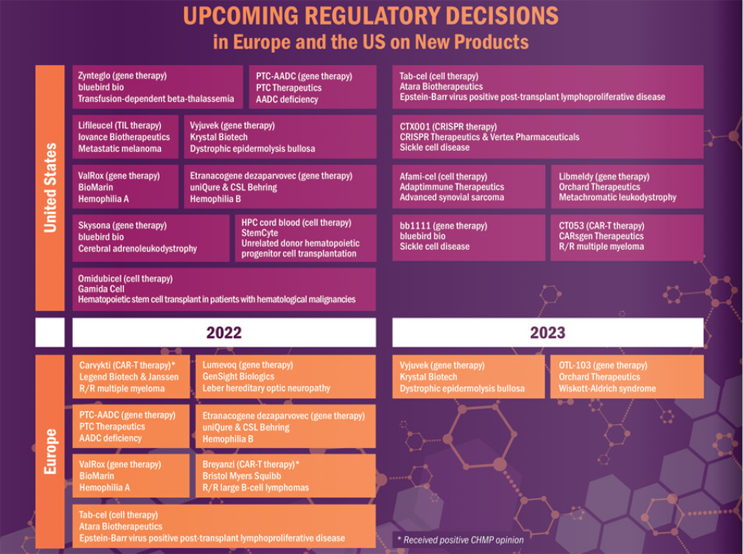
Figure 4: Upcoming regulatory decisions in the US and EU for new advanced therapies.5
Reasons to be optimistic
The growth in the number of clinical trials with ATMPs highlights both the industry and the regulatory agencies’ confidence in the science that underpins these treatments. To be able to have reached the clinic, there has already been a significant amount of work undertaken to demonstrate that not only are these interventions safe to be administered, but they also have a high probability of demonstrating clinically meaningful efficacy in the disease indication and show benefit over existing therapies.
There have been understandable questions about the longevity of gene and cell therapies, but recent data demonstrating the persistence of CAR-T cells for up to a decade2, and significant reduction in annualised bleeding rate in haemophiliacs over 18 months6, has helped to assuage these concerns. Furthermore, data demonstrating a median follow up of 17 months for patients with sickle cell disease essentially represents a functional cure for patients, providing them with a quality of life free from severe disease events for many months.7 Together, these clinical results demonstrate durability of efficacy and therefore give hope to patients and their families.
The UK has been at the forefront of approving new advanced therapies, being one of the first countries to approve the commissioned use of CAR-T therapies in 20188 as well as treatment for spinal muscular atrophy in 20219. Across the UK, there are now a significant number of hospitals delivering commissioned ATMPs to patients, as shown in Figure 5.
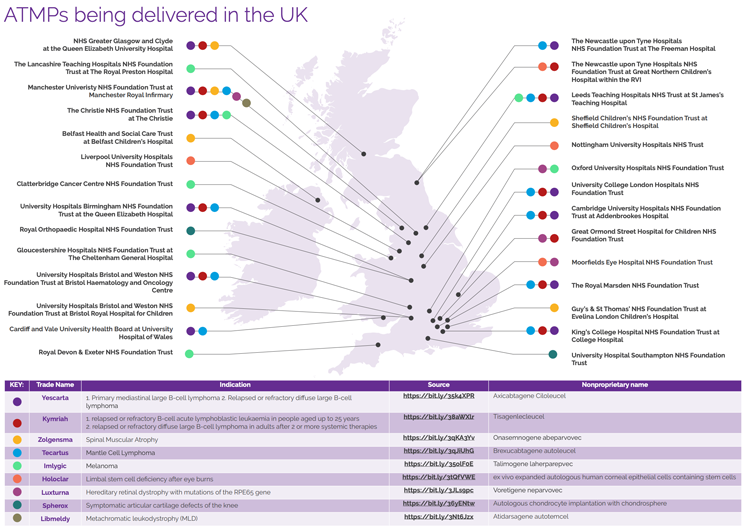
Figure 5: Commissioned ATMPs being delivered to patients across the UK.10
With a focus on manufacturing sector skills, the UK Cell and Gene Therapy Catapult has recently reported that by 2026, there will be up to 10,000 bioprocessing jobs in the UK ATMP sector - an increase of 6000 from the 2021 figure - with a total workforce of 15,00011, representing an increase of 151% compared to that of today. It is expected that this workforce will come from a range of backgrounds, including experienced staff within the wider biotech industry, recent graduates, and from the extremely successful ATAC (Advanced Therapies Apprenticeship Community).12 The ATAC scheme has been established to help develop the manufacturing workforce and has placed 137 apprentices across 37 companies, all working towards qualifications as well as gaining valuable hands-on experience making ATMPs.13
How the UK is responding to ATMP challenges
Running clinical studies for advanced therapies can be technically challenging. Not only do many of these therapies have little precedent to follow, but it may also be the case that there is a general lack of understanding at a local level as to what advanced therapies are or why they are different to conventional small or large molecules. At the International Society for Cell and Gene Therapy meeting in 2022, there was a presidential call to universally address education and training in working with ATMPs for everyone involved in the clinical trials process, from bench to bedside.14
One thing people need to remember is that the fundamentals of clinical trial operations are the same, irrespective of the modality of the treatment, be it small or large molecule, cell or gene therapy. There are now educational resources available online to help educate clinical staff about advanced therapies, which help to reduce the ‘fear of the unknown.’ In the UK, Health Education England has partnered with other groups to develop a freely available series of modules that can be found here. The American Society for Gene and Cell Therapy has also developed a series of engaging animations, available here.
Once the education hurdle has been overcome and teams become more familiar with the new way in which advanced therapies work with the body to modify diseases, fear of the unknown will be reduced, and we will be able to focus on the delivery of these treatments.
In the UK, there has been some excellent sharing of best practice led by the Advanced Therapies Treatment Centre (ATTC) programme, designed to allow hospitals to rapidly set up the services required for the delivery of ATMPs to patients. This resource is open access and can be found here. Examples of the types of documentation available include governance structures, pharmacy SOPs, patient treatment pathways, and training resources.
The ATTC programme has built a network of hospitals and experienced researchers across the UK to help rapidly embed advanced therapies into routine clinical practice. This has delivered significant gains to the UK through shared learning, developing standards that can be applied globally, and increasing patient access to ATMPs.
The Association of British Pharmaceutical Industry (ABPI), as a partner in the Advanced Access collaborative (AAC) along with other partners such as the ATTC network, MHRA, NICE and other stakeholders, has produced a roadmap to help companies navigate the development and regulatory pathway15. This is an interactive resource which helps to answer many of the questions developers of advanced therapies may have about who to speak to and what activities need to be carried out at different stages of the development process.
The UK Cell and Gene Therapy Catapult exists to support the development of the ATMP industry and has recently published a vision for the UK, to build on the success of the ATTCs 16. Within this, they call for a series of connected actions in a number of different areas, including:
- Horizon scanning – to better understand upcoming treatments and allow planning for these to take place.
- Patient data – harness the potential of the NHS to understand the effectiveness of treatments and facilitate alternate reimbursement approaches.
- Capacity increase – the expected influx of ATMPs into commissioned services will increase demand on certain services such as apheresis, now is the time to plan effectively.
- Patient care and support – patient education about these treatments is essential, involving patients in the design of support services and education significantly helps here.
- Workforce planning – Growth in ATMPs requires a skilled workforce to be in place to deliver them, providing a structured syllabus for staff to follow helps to build this knowledge.
- Alternative reimbursement approaches – exploring models that allow the NHS to pay for ATMPs over time can ensure value for money is gained from these expensive treatments.
Activities across Europe
In Sweden a small-scale version of the ATTC programme called ATMP Sweden has been operating, which has produced a number of guides and documents aimed towards sponsor companies, available here. This resource includes Good Manufacturing Practice (GMP) checklists, investigator brochure guides, and an Investigational Medicinal Product Dossier guide, all with specific tips on what should be included for ATMP development. No other countries across Europe are operating a coordinated process to support the development of the ATMP industry.
Summary
The number of companies developing new advanced therapies and progressing these into and through clinical trials worldwide and in the UK is encouraging. Equally, the number of approvals of ATMPs is rising steadily and looks to continue to do so in the near future. Collectively, this equates to a positive outlook for patients, especially as many ATMPs address rare diseases/conditions that are not effectively treated at present. Companies developing ATMPs and hospitals delivering them can now access a significant number of resources that allow them to learn from others, to reduce the time taken from bench to bedside. In the UK, the ATTC network, the ATAC scheme, and the AAC have all been instrumental in accelerating the development and adoption of ATMPs within the country and are excellent examples of how governments can pull together resources to accelerate the delivery of new medicines to patients.
Boyds has significant experience in overcoming the challenges faced by developers of ATMPs. We have supported seven of the currently licensed ATMPs along their journey through the clinic as well as many more currently in clinical development. The experience of drug development consultants well-versed in the challenges of developing advanced therapies is essential to ensure their efficient development and facilitate patient access to these life changing medicines.
About the author
 Ian Hollingsworth M.Sc. is associate director of product development at Boyds, a leading pharmaceutical and biotech product development consultancy.
Ian Hollingsworth M.Sc. is associate director of product development at Boyds, a leading pharmaceutical and biotech product development consultancy.
Ian is PMP (Project Management Professional) qualified and has over 20 years’ experience in the pharmaceutical and biotech industries, working in big pharma before moving into project managing early-stage programmes. Ian has managed projects in production facilities as well as transnational drug development programmes, including the UK initiative to establish Advanced Therapy Treatment Centres (ATTC) across the NHS.
Boyds was established in 2005 by Professor Alan Boyd and provides specialist consultancy services to an international client base, supporting the development of new, cutting-edge medicines for patient benefit.
Working with early stage biotechs, pharma companies, medical device companies and universities, the team supports the development of advanced therapy medicinal products (ATMPs), small molecules and biologics across a wide range of therapeutic areas.
The team has expertise in programme management and product development, clinical operations, medical monitoring, regulatory affairs and strategy, medical writing and provides scientific and medical advice to help translate ideas into medicines.
For more information, please visit: www.boydconsultants.com
References
- https://ct.catapult.org.uk/clinical-trials-database
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9166916/
- https://alliancerm.org/
- https://ct.catapult.org.uk/
- https://alliancerm.org/sector-report/2021-annual-report/
- https://www.hemophilia.org/news/uniqure-and-csl-behring-announce-trial-updates-for-hemophilia-b-gene-therapy
- https://www.nejm.org/doi/full/10.1056/NEJMoa2117175
- https://www.nice.org.uk/news/article/nice-recommends-cutting-edge-therapy-for-young-people-with-blood-cancer
- https://www.nice.org.uk/guidance/hst15
- https://attc-143fd.kxcdn.com/wp-content/uploads/2022/03/Final-version-map-and-calendar-combined.pdf
- https://ct.catapult.org.uk/sites/default/files/publication/2021%20Skills%20Demand%20Survey%2
0Report%20-FINAL_TO%20PUBLISH.pdf - https://advancedtherapiesapprenticeships.co.uk/
- https://fr.zone-secure.net/64109/.Catapult-Annual-Review-2021/#page=1
- https://www.scienceboard.net/index.aspx?sec=rca&sub=isct_2022&pag=dis&itemid=4228
- https://www.abpi.org.uk/publications/advanced-therapy-medicinal-products-atmps-roadmap-tool/
- https://ct.catapult.org.uk/sites/default/files/publication/National%20Cell%20and%20Gene%20
Therapy%20Vision%20for%20the%20UK.pdf
Caption for image supplied in support of this article: Ian Hollingsworth, associate director of product development at Boyds
For further information, please contact: Sue Carr on 07809 727533, email: sue.carr@boydconsultants.com
Notes to editors:
About Boyds
Boyds was established in 2005 by Professor Alan Boyd and provides specialist consultancy services to an international client base, supporting the development of new, cutting-edge medicines for patient benefit.
Working with early stage biotechs, pharma companies, medical device companies and universities, the team supports the development of advanced therapy medicinal products (ATMPs), small molecules and biologics across a wide range of therapeutic areas.
The team has expertise in programme management and product development, clinical operations, medical monitoring, regulatory affairs and strategy, medical writing and provides scientific and medical advice to help translate ideas into medicines.
Boyds is headquartered in Crewe, UK, with offices in Cambridge, Dublin, and Pennsylvania. The company opened its US office in 2021, in response to increased US demand from its global client base.
In 2018, Boyds was awarded the prestigious Queen’s Award for Enterprise, International Trade in recognition for its international work, and in particular its support in the development of gene therapies for the treatment of rare diseases.
Boyds is the trading name for Alan Boyd Consultants Limited in the UK, Boyds Consultants Limited in Ireland, and Alan Boyd Consultants Inc in the USA.
For more information, please visit www.boydconsultants.com
