Addressing The "Achilles Heel" Of Gene Therapy Manufacturing
By Andrew Knudten, Chief Operating Officer, Jaguar Gene Therapy

By treating the underlying genetic cause of a disease, gene therapy is poised to move the healthcare pendulum from symptom management to transformational therapy. But as Peter Marks, the director of the Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research (CBER), and Ana Hidalgo-Simon, head of advanced therapies at the European Medicines Agency (EMA), recently described at the American Society of Gene and Cell Therapy (ASGCT) 2022 Annual Meeting, manufacturing is a key hurdle preventing these potentially transformative therapies from getting to patients.
Achieving excellence in CMC (Chemistry, Manufacturing and Controls) is the most important factor in moving the gene therapy field forward. At Jaguar Gene Therapy, we have assembled one of the most experienced CMC teams in the field to set a new bar on CMC expectations commercially and in the clinic. With our cross-functional knowledge and successful prior experience manufacturing an approved gene therapy, we are creating new and innovative CMC solutions for gene therapies. We are thoughtfully and purposefully propelling the industry forward to ensure we bring the safest and most effective gene therapies to patients and families.
At Jaguar, we’re focusing on three strategic CMC priorities we believe will allow us to accelerate our own gene therapy programs and ultimately help advance the entire field.
Ensure the CMC Package for INDs is Robust
The “Achilles heel” of gene therapy is suboptimal CMC packages for Investigational New Drug (IND) submissions. The U.S. FDA is urging innovators to generate and share their data, advance their thinking, and push the boundaries beyond the minimum requirements for these applications.
To achieve this, innovators must develop substantial experience with their full-scale process, analytics, and compliance with Good Manufacturing Practices (GMP) prior to IND submission to fully understand the impurity profile that is the result of their process. This cannot be achieved with shaker flask experiments or running a handful of 5 or 10 liter batches. Fast and cheap is not the answer. Innovators must generate a robust body of data in order to fully understand their process capabilities and define the critical quality attributes of their important gene therapy medicines.
Pursue a Commercially Viable Process from the Start
CMC is the most important technical asset for an early-stage gene therapy company. Regulators will not, and should not, give latitude to companies around the comparability and quality of the material used at different development stages because it has a major impact on safety and efficacy. By developing a commercially viable manufacturing process at the start, a company can reduce the likelihood bridging studies will be required. We have observed many examples in the field where product quality at a small-scale is vastly different from large-scale. Hoping for “leniency” or reduced expectations is not a fair request to make to regulators. More importantly, asking patients receiving the investigational or commercial product to take a medicine of lesser quality is unacceptable.
Continuity in manufacturing throughout the product lifecycle is crucial. By not investing early in manufacturing, companies have run into roadblocks where the investigational products they studied very early on were significantly different from those studied later in the development process, leading to delays and unforeseen expenditures. A scalable process with a consistent formulation also allows for a seamless supply, a challenge that has become even more severe during the pandemic. Innovators must always strive to improve the process and partner with regulatory experts to accurately track and report any process differences. With an appropriate risk assessment, most minor changes that result in a purer product can be discussed and implemented. If changes are significant that may reduce the purity or add risk to the process or product, that’s where comparability enters the equation, which is not an ideal situation.
At Jaguar, we believe internal CMC control is a strategic capability. Our experienced CMC team is committed to innovation to create new processes. With the announcement of our new GMP manufacturing facility last November, we have taken a significant step in preparing for commercial demand in the years ahead. Once this 174,000-square-foot facility is fully converted to Jaguar’s gene therapy manufacturing specifications, it will support future clinical and commercial production for our initial programs and beyond.
Raise the Bar on Purity and Potency
The industry recognizes it is imperative to deliver safe gene therapy solutions. At Jaguar, we aim to be on the leading edge of producing the purest and most potent gene therapy products because we understand lives are at stake.
Elevated empty capsid levels continue to lead to safety concerns because of their potential to produce an immune response without providing any therapeutic benefit. Dark Horse Consulting announced draft guidance (Testing of Adeno-Associated Viral (AAV) Vector-Based Human Gene Therapy Products for Empty Capsids During Product Manufacture) suggesting that empty capsid levels of <30% are acceptable. We believe we must do a lot better.
Technology provides us with the opportunity to generate a more potent and purer product through biochemistry techniques and advanced analytics. Gene therapy companies that rely on standard or minimal analytics consequently may select the incorrect product dose for their clinical trials or worse yet, miscalculate the actual dose or purity of their product. This may result in patients receiving a dose that is therapeutically suboptimal and potentially unsafe. The importance of advancing analytical methods very early in the process to fully understand the product’s critical quality attributes should not be underestimated. The standards should be higher, and we can no longer try to move gene therapies into the clinic or submit an IND with the bare minimum data set.
At Jaguar, our process for identifying and selecting for “functional full” capsids will help increase confidence in the scientific field and marketplace in delivering safe gene therapies. We enrich for “functional full” capsids to maximize purity, enabling definitive separation of therapeutically active and partial capsids well beyond what typical column chromatography can achieve. Less than 30% empty capsids with additional partial sequence capsids are not good enough.
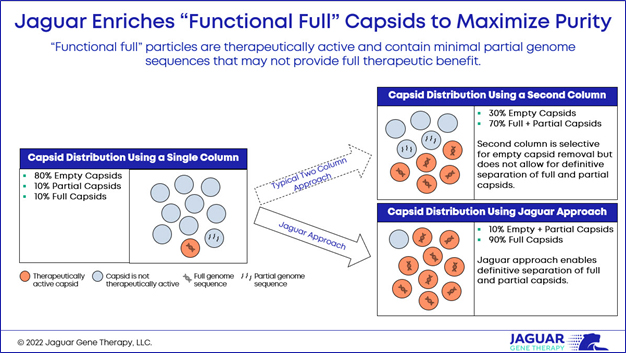
If our process improves overall purity, we believe it will lead to improved safety and the ability to utilize gene therapy-based approaches in larger patient populations. That also means with a higher purity profile, we could potentially use a smaller dose, resulting in a safer product and lower cost of goods.
The bottom line: regulatory authorities expect all innovators in the gene therapy field to advance their thinking and not just meet the minimum requirements. To achieve this, experience matters. An industry-leading CMC package and team is the fundamental success factor enabling all vector technologies. At Jaguar, our approach is grounded in our experience but pushes beyond it to deliver the purest and most potent gene therapy products possible. That is what every patient deserves.
# # #
Andrew Knudten Bio
 About: Andrew Knudten is the Chief Operating Officer of Jaguar Gene Therapy, a company uniquely positioned to accelerate breakthroughs in gene therapy for patients suffering from severe genetic diseases. He has extensive experience developing and successfully commercializing gene therapy treatments. With an M.S. in cell biology and genetics, he has expertise in operations, manufacturing, process development and improvement.
About: Andrew Knudten is the Chief Operating Officer of Jaguar Gene Therapy, a company uniquely positioned to accelerate breakthroughs in gene therapy for patients suffering from severe genetic diseases. He has extensive experience developing and successfully commercializing gene therapy treatments. With an M.S. in cell biology and genetics, he has expertise in operations, manufacturing, process development and improvement.
Before joining Jaguar, Andrew served on the executive team at AveXis during the development and approval of Zolgensma®. Prior to that, he also served in executive leadership roles at Cirius Therapeutics, Hospira, Novartis, CoDa Therapeutics and Amgen.
