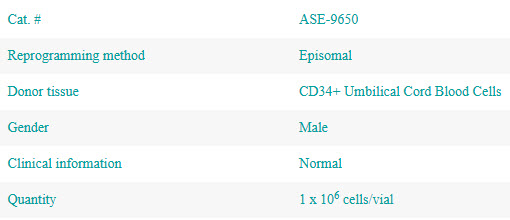
TARGATT™ Hypo hiPSC Knock-In Kit

Rapidly advance your allogeneic development with a hypoimmunogenic iPSC platform that has pre-built TARGATT™ large knock-in technology.
- Favorable development profile—reprogrammed from CD34+ cord blood cells for reduced immunogenicity and fewer somatic mutations compared to reprogrammed adult cells
- Hypoimmunogenic—B2M/CIITA double knock-out eliminates expression of HLA Class I and II molecules to avoid recognition by CD8+ and CD4+ T cells
- Large knock-in ready—quickly add additional immune evasion and disease modifying genes at the reliable H11 safe harbor locus using TARGATT large knock-in technology
- Excellent differentiation potential—we’ve successfully differentiated the parental line into a number of cell types, including cardiomyocytes, NK cells, T cells, monocytes/macrophages, and endothelial cells
- Commercialization-ready—genome editing performed with Mad7 for a single, cost-effective license and clear freedom to operate
Overview
Reprogrammed from CD34+ cord blood cells and rendered hypoimmunogenic by knocking out both the β2 microglobulin (B2M) gene and the HLA class II transactivator (CIITA) gene, the ActiCells™ RUO TARGATT™ Hypo hiPSC Knock-in Kit (Cat. # AST-9650) is a fast and reliable way to build your allogeneic cell-based medicine.
Designed to jumpstart your allogeneic development, the ActiCells™ RUO TARGATT™ Hypo hiPSC Knock-in Kit gives you a hypoimmunogenic cell line that is immediately ready for further modification. Knock in additional immune evasion elements to avoid clearance by NK cells, macrophages, and dendrites while also expressing disease-modifying genes. The power of the TARGATT™ platform means you can insert all these genes in a single reaction—up to 20 kb—with additional DNA over 20 kb added in nested reactions.
The other advantage of TARGATT™ technology lies in its placement at the H11 safe harbor locus, which avoids the potential for silencing and harmful random integration that you get with lentivirus and transposon-mediated integration and consistently delivers gene expression.*
ActiCells™ RUO TARGATT™ Hypo hiPSCs will be the research-matching isogenic cell line to the ActiCells™ GMP TARGATT™ Hypo hiPSCs which are in development.
Don’t need TARGATT™ technology? We also offer ActiCells RUO Hypo hiPSCs (Cat.# ASE-9550) as a cell line only product.
Each ActiCells™ RUO TARGATT™ Hypo hiPSCs Knock-in Kit contains sufficient reagents for 3 transfections and includes:
- AST-9650-C ActiCells™ RUO TARGATT™ Hypo hiPSCs
- AST-3091 TARGATT™ 46 CAG-MCS Cloning Plasmid
- AST-3084 TARGATT™ CAG-Integrase Plasmid
Every lot is tested for viability following recovery from cryopreservation, functional pluripotency (formation of the three germ layers), expression of pluripotency markers (OCT4, SOX2, NANOG, SSEA-4, and TRA-1-60), presence of alkaline phosphatase (AP), STR, sterility, and for the absence of mycoplasma and pathogens (CofA available upon request).
*Constructs must have already been shown to be expressable in iPSCs or the differentiated cell type.
How it works
Cell therapy rejection is, in part, mediated by CD8+ T cells (cytotoxic T cells), which interact with HLA class I molecules, and CD4+ T cells (helper T cells), which interact with HLA class II molecules (Figure 1).
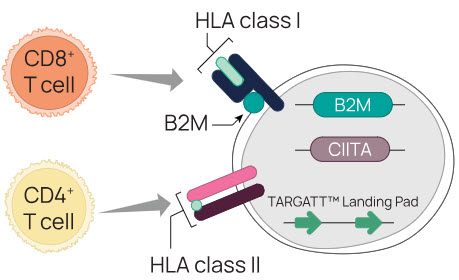
Figure 1. Avoiding the host T cell response is critical for overcoming allograft rejection.
To make hypoimmunogenic hiPSCs, we eliminated cell surface expression of HLA I and HLA II molecules by knocking out two genes (Figure 2):
- B2M, which encodes the ß2-microglobulin protein, a key part of the HLA class I complex
- CIITA, the HLA II transactivator which is essential for transcription of HLA class II genes
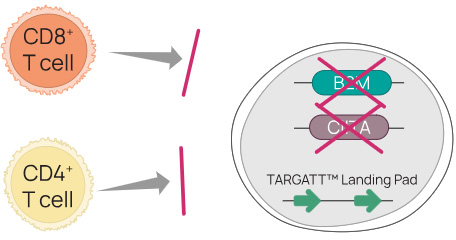
Figure 2. Knocking out cell surface expression of HLA I and HLA II eliminates the T cell response, although other gene edits are needed to avoid innate immune responses. To eliminate cell surface expression of HLA I and HLA II we created a homozygous double knock-out of the B2M and CIITA genes in a hiPSC line containing a TARGATT landing pad.
Learn more about our approach to developing hypoimmunogenic hiPSCs by watching our webinar, “The Future of Allogeneic Therapy: ASC’s Hypoimmunogenic Donor Cells.”
